Serum albumin binding proteins with long half-lives
A serum albumin and half-life technology, applied in the direction of prolonging plasma life fusion, anti-animal/human immunoglobulin, immunoglobulin, etc., can solve the problems of reducing patient compliance and increasing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
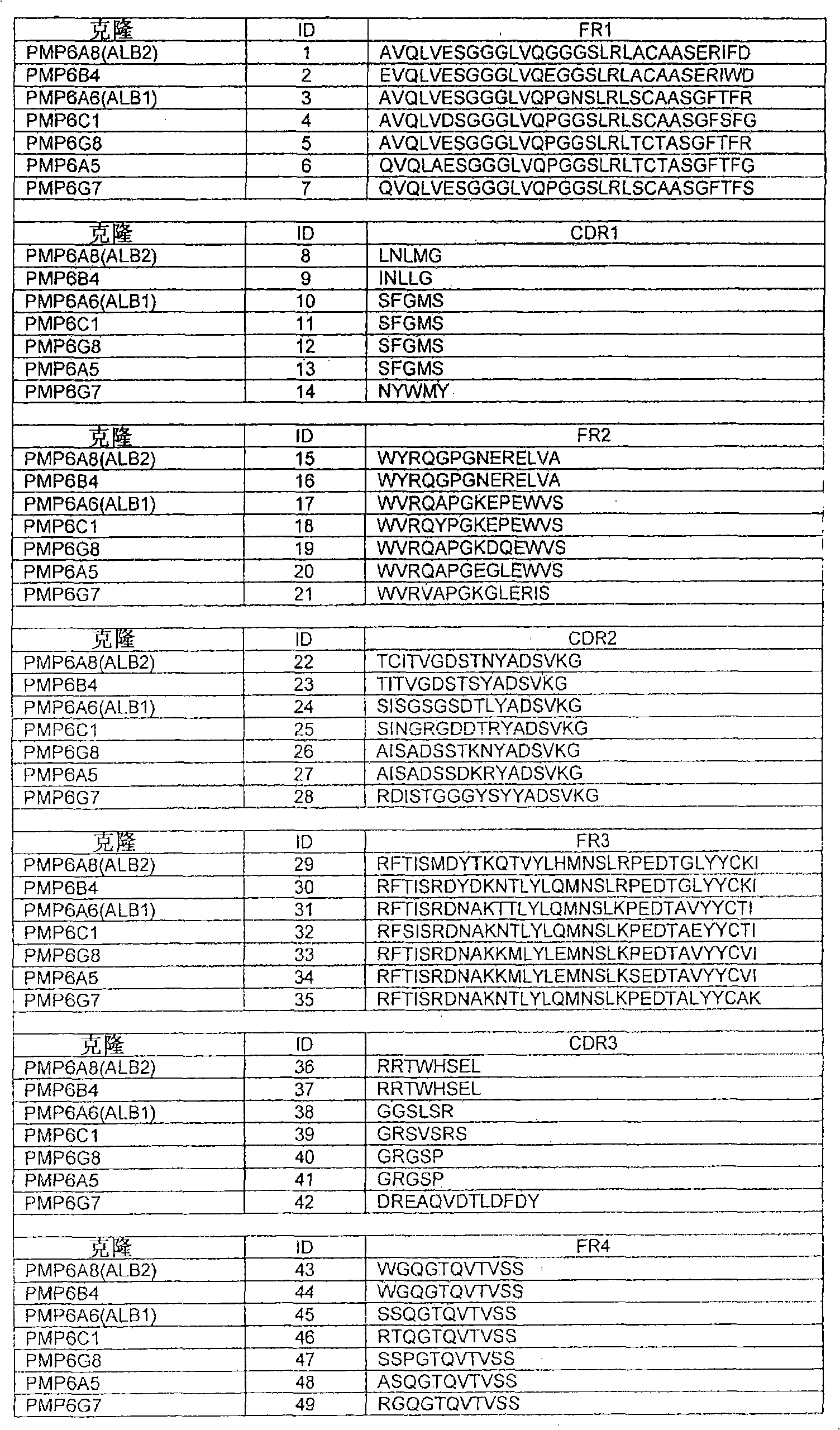
[0201] Example 1: Identification of Nanobodies Specific for Serum Albumin
[0202] Identification of albumin-specific Nanobodies from camels immunized with human serum albumin. Screening of various Nanobodies is performed by ELISA using human, macaque and mouse albumin, thereby generating a set of Nanobodies that cross-react with serum albumin of different species.
Embodiment 2
[0203] Example 2: Biacore analysis
[0204] The binding of Nanobodies to serum albumin was characterized by surface plasmon resonance in a Biacore 3000 instrument. Serum albumin from different species is covalently bound to the surface of the CM5 sensor chip through amine coupling until an increase of 250 response units is achieved. Inactivate the remaining reaction group. The binding of Nanobodies was evaluated at one concentration (1:20 dilution). Each Nanobody was injected at a flow rate of 45 μl / min for 4 minutes, allowing it to bind to the antigen bound to the chip. At the same flow rate, the Nanobody-free binding buffer was flowed through the chip for 4 hours to allow the bound Nanobody to dissociate spontaneously. Calculate K from sensorgrams obtained for different Nanobodies off -value. Follow K off -Value, for the rating of the tested Nanobody, see Table IV below:
[0205] Table IV:
[0206] Kind
People
Mouse
C
PMP6A8
PMP6A8
PMP6B4
C
PMP6B4
...
Embodiment 3
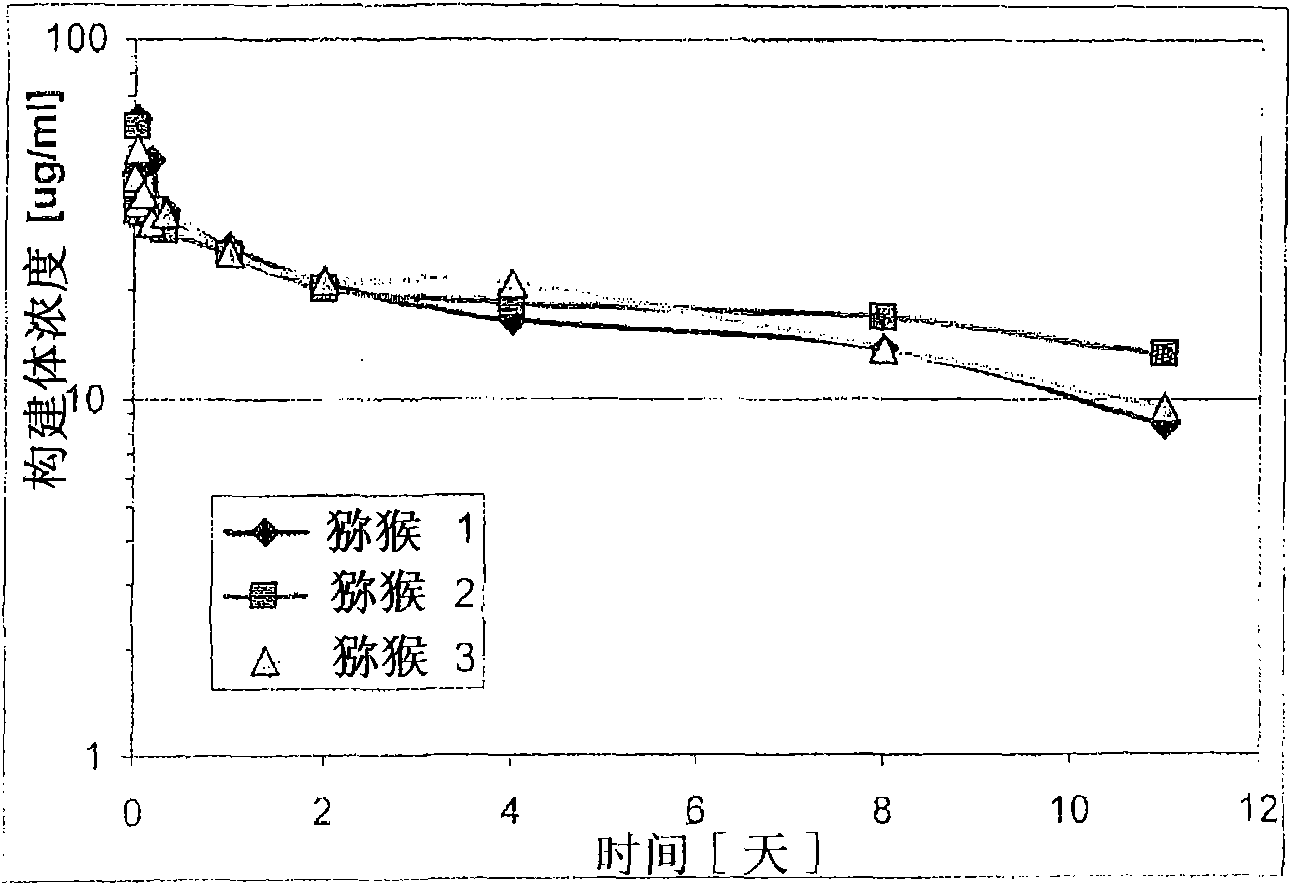
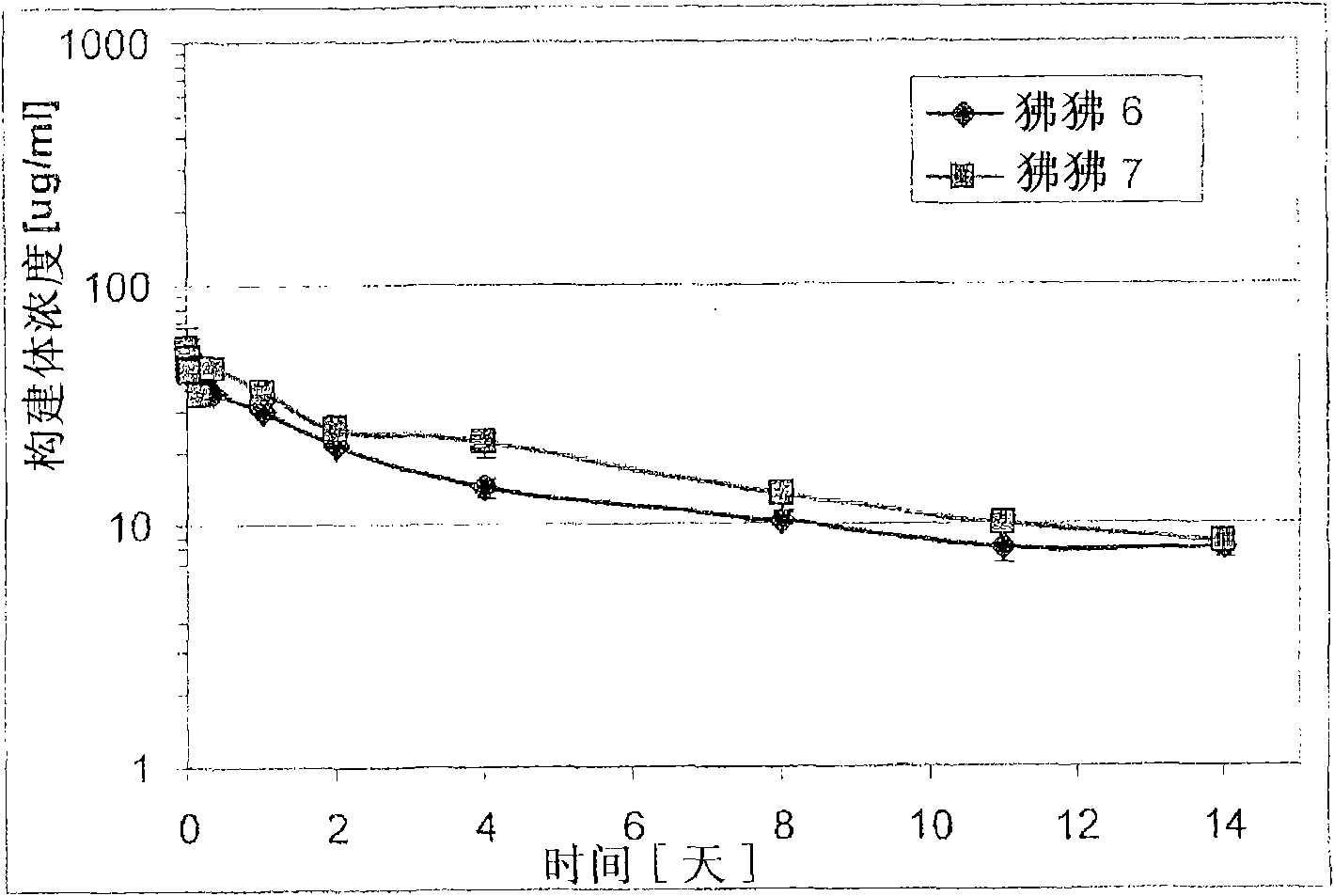
[0212] Example 3: Half-life in rhesus monkeys
[0213] The pharmacokinetic characteristics of trivalent bispecific nanobody constructs including humanized anti-human serum albumin nanobody ALB-8 (SEQ ID NO: 62) were studied in rhesus monkeys. On day 0, three monkeys received the construct at 2 mg / kg. Immediately after application and at 1, 2, 4, 8, 11, and 14 days after application, plasma samples (shown below) were obtained from the rhesus monkeys and analyzed to determine pharmacokinetic characteristics. The PK characteristics in all monkeys are similar, with a calculated half-life of about 10 days. The calculated half-life is within the range of the estimated half-life of macaque albumin.
[0214] Before the environmental adaptation study, the three monkeys were adapted for 4 weeks. On day 0, the monkey was instilled intravenously into the right or left vena cephalica by using an indwelling catheter and an infusion pump, and received the construct at 2 mg / kg. The dose was ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com