Protein biomarker for assisting in authenticating Rituximab drug-resistant ABC-DLBCL cells and application thereof
A protein marker and marker technology, applied in animal cells, tumor/cancer cells, vertebrate cells, etc., can solve the problems of poor prognosis and the inability to obtain complete remission with conventional second-line regimens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1, the construction of drug-resistant cell line
[0076] Culture conditions: 37°C, 5% CO 2 in the cell culture incubator.
[0077] 1. The SU-DHL-2 cell line was cultured in the RPMI-1640 medium containing 10% fetal bovine serum to the logarithmic growth phase.
[0078] 2. After completing step 1, centrifuge at 1000 rpm for 5 minutes, discard the supernatant, add new RPMI-1640 medium containing 1 μg / mL Rituximab and 10% fetal bovine serum, and incubate for 24 hours.
[0079] 3. After completing step 2, centrifuge at 1000rpm for 5min, discard the supernatant, add new RPMI-1640 medium containing 10% fetal bovine serum, and culture the cells to the logarithmic growth phase.
[0080] 4. After completing step 3, centrifuge at 1000 rpm for 5 minutes, discard the supernatant, add new RPMI-1640 medium containing 1 μg / mL Rituximab and 10% fetal bovine serum, and incubate for 24 hours.
[0081] 5. After completing step 3, centrifuge at 1000rpm for 5min, discard the s...
Embodiment 2
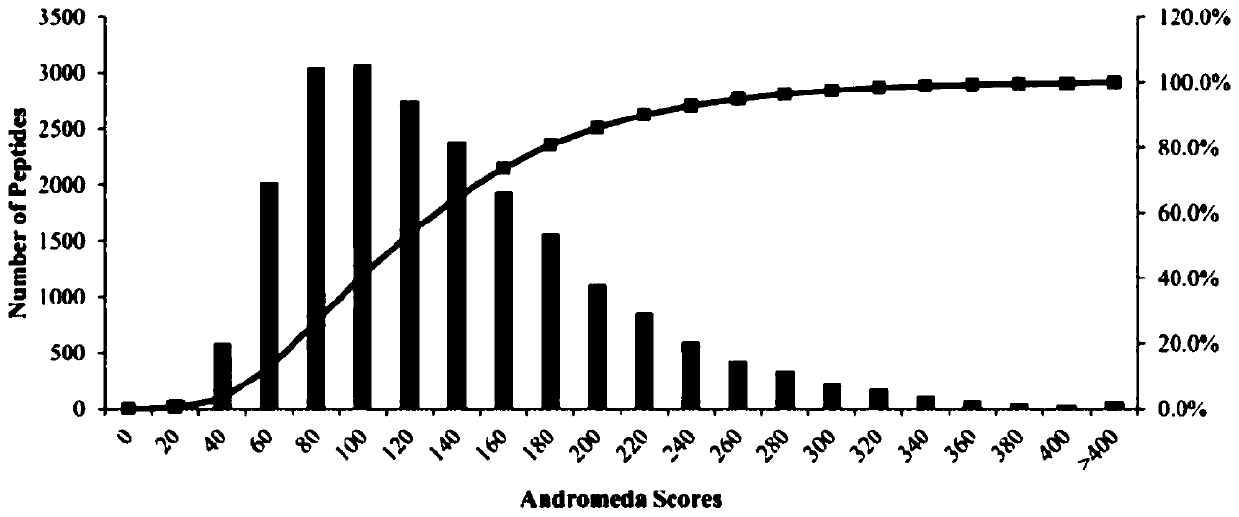
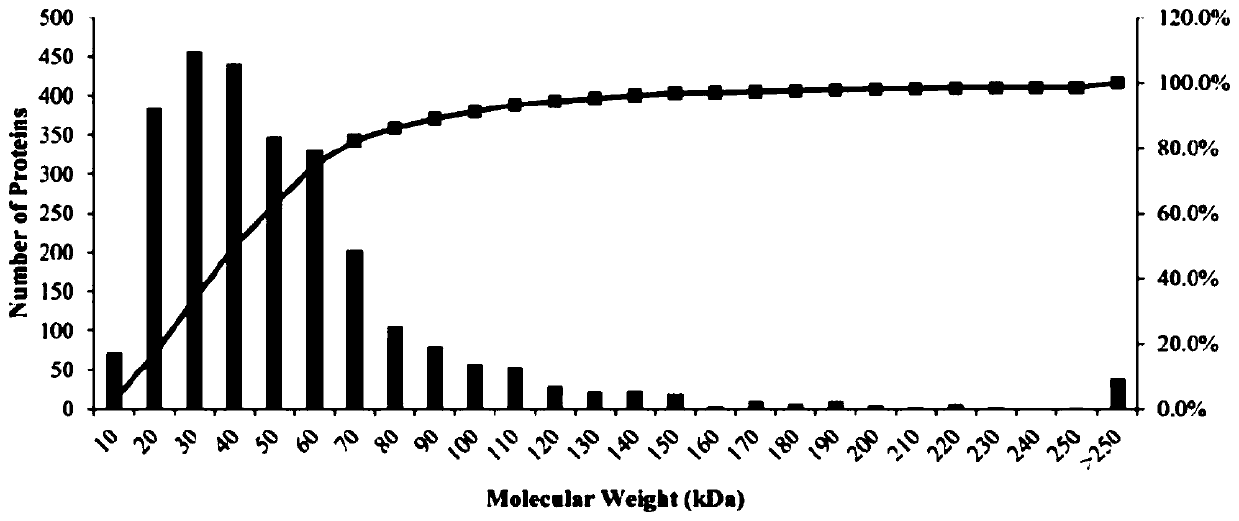
[0106] Embodiment 2, label-free quantitative proteomics (Label-free) method
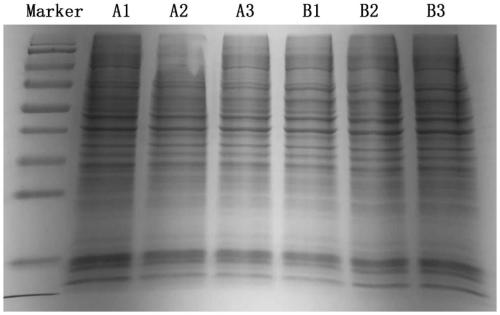
[0107] The cell samples are: cell sample A1, cell sample A2, cell sample A3, cell sample B1, cell sample B2, and cell sample B3. Cell sample A1, cell sample A2, and cell sample A3 are all SU-DHL-2-WT, representing 3 biological replicates. Cell sample B1, cell sample B2, and cell sample B3 are all SU-DHL-2-R, representing 3 biological replicates.
[0108] 1. Preparation of Protein Solution
[0109] Take cell samples, add SDT lysate, sonicate (80W, work for 10s, pause for 15s, cycle 10 times), then boil in water bath for 15min, then centrifuge at 14000g for 40min, take the supernatant, which is the protein solution.
[0110] SDT lysate: 4g / 100ml SDS, 100mM Tris-HCl, 1mM DTT, pH7.6.
[0111] Protein quantification was carried out by BCA method (BCA quantification kit, P0012, Biyuntian). Aliquot the samples and store at -80°C.
[0112] 500 μL protein solution was obtained for each cell sample. Acco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com