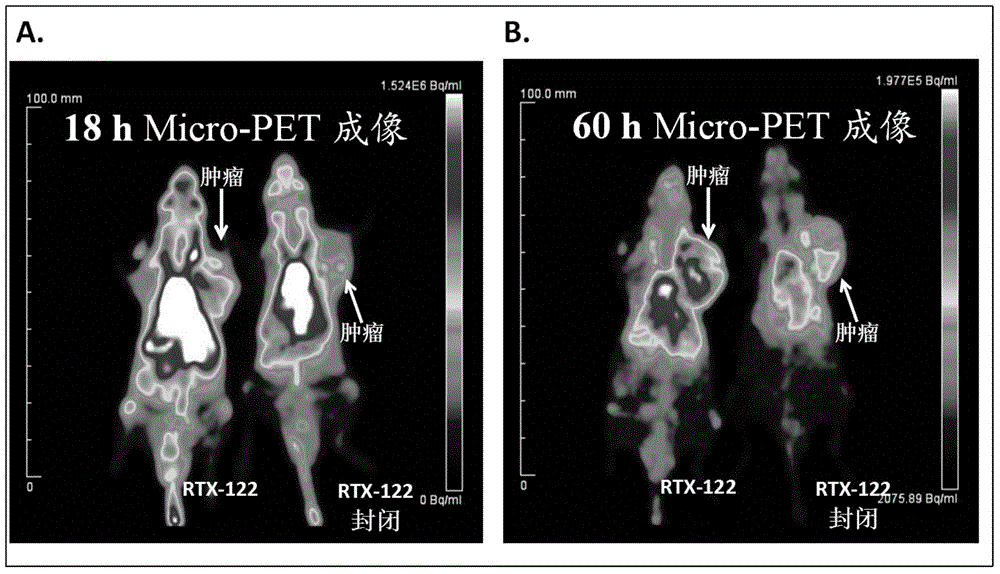
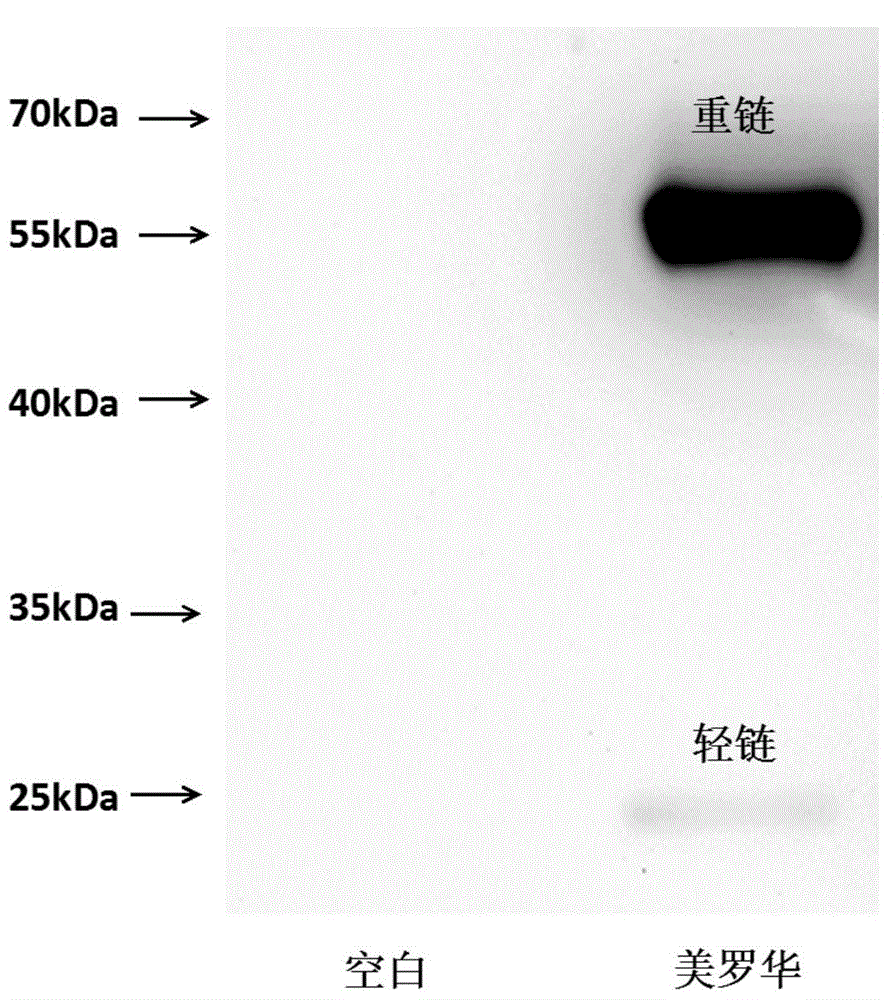
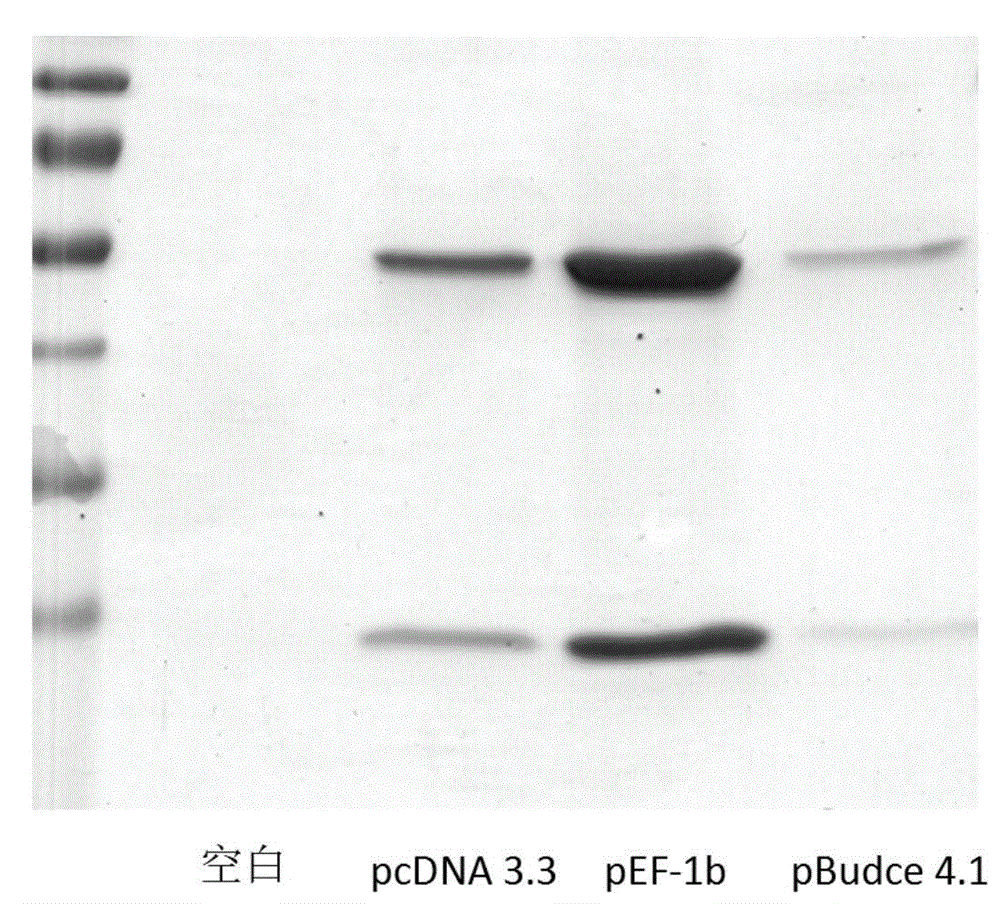
Novel unnatural amino acid marked antibody-drug conjugate and preparation thereof
A technology of unnatural amino acids and amino acids, which is applied in the field of fixed-point coupling of small molecule drugs, can solve the problems of increasing the immunogenicity of antibody drugs, reducing the half-life of antibodies, and affecting the binding of antibody antigens, achieving simple and easy coupling reactions, reducing The possibility of off-target, the effect of reducing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1: Construction of the gene vector comprising the rituximab of site-directed mutation
[0099] (1) Construction and acquisition of auxiliary plasmids
[0100] Through the optimization and experimentation of the conditions, it was determined that the tandem expression of the four tRNAs from the 7sk promoter (its sequence is shown in SEQ ID NO: 6) was optimal, and pXH-N was constructed and obtained 3 Auxiliary vector: the vector uses pUC19 as a template, and introduces four tandem 7sk promoters and tRNA by using the BamHI restriction site; then introduces tRNA synthetase and its poly A termination signal under the control of the CMV strong promoter; uses EcorI Restriction sites are introduced into eukaryotic replication originals f1 ori and SV40; the plasmid can express tRNA and tRNA synthetase that specifically recognize the unnatural amino acid NAEK.
[0101] (2) Obtaining the plasmid containing rituximab
[0102] The genes of rituximab (SEQ ID NO: 1 and ...
Embodiment 2
[0111] Example 2: Expression and purification of rituximab for site-directed mutation
[0112] Construct pXH-N in the present invention 3 The plasmid contains tRNA (tRNA Pyl ) and pyrrolysyl-tRNA synthetase (pheRS), in expressing cells, the amber stop codon (TAG) is used as the sense codon, which can make the non-natural amino acid NAEK incorporated into the protein, thus causing the site-specific expression of rituximab mutation. Next, the inventors examined the incorporation possibility of NAEK and the production performance of the mutant protein.
[0113] 1: Synthesis and identification of unnatural amino acid NAEK
[0114] The chemical synthesis reaction formula of unnatural amino acid Lys-azido is as follows
[0115]
[0116] As described in the above formula, 2.3 mL of raw material 1 (2-bromoethanol) was dissolved in a mixed solution of 90 mL of acetone and 15 mL of water, and 3.12 g of NaN3 was added, and heated to reflux in an oil bath at 60°C for 20 h. Cool ...
Embodiment 3
[0131] Example 3: Site-directed coupling of mutants to a bifunctional tether (DIBO-DOTA)
[0132] 1: Synthesis and identification of bifunctional linker DIBO-DOTA:
[0133]
[0134] Compound 1 (2.88g, 14.0mmol) was dissolved in 20mL of anhydrous CH2Cl2, under N2 protection, BF3 OEt2 (2.59mL, 21.0mmol) was slowly added, then the system was moved to a cold well at -10°C, and slowly added dropwise under stirring A CH2Cl2 solution of trimethylsilylated diazomethane (2.0 mol / Lin hexanes) (10.5 mL of trimethylsilylated diazomethane dissolved in 20 mL of anhydrous CH2Cl2) was added dropwise within 1 hour. After continuing to react at -10°C for 2-4 hours, the reaction solution was poured into 50 mL of ice water to quench the reaction, the organic phase was separated, and the aqueous phase was extracted with CH2Cl2 (2×50 mL). The organic phases were combined and washed with saturated brine (2×40mL), dried over anhydrous sodium sulfate, concentrated by filtration, and separated on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com