Methods of treating cancer using IL-21 and monoclonal antibody therapy
An IL-21, monoclonal antibody technology, applied in the direction of antibody medical components, chemical instruments and methods, antibodies, etc., can solve the problem of not being able to stimulate human immune effector cells, human anti-mouse, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] IL-21 enhances antibody-dependent NK cell activity
[0104] a.
[0105] Peripheral blood was obtained, and mononuclear cells (MNC's) were prepared by ficoll centrifugation. Using StemSep TM Human NK Cell Stem CellTechnologies (Vancouver, British Columbia) human NK cell negative enrichment kit, natural killer (NK) cells were purified from MNC populations by negative enrichment. Briefly, MNCs were labeled with lineage-specific antibodies (excluding NK lineages), which were in turn magnetically labeled. The labeled MNCs are then passed through a magnetic column, where labeled cells are retained, while unlabeled NK cells flow through the column.
[0106] at 5x10 5 Density of cells / mL NK cells were plated in αMEM / 10% autologous serum / 50 μM β-mercaptoethanol and 0, 1, 10 or 100 ng / mL hIL-21 (A794F) or 10 ng / mL IL-12 (positive control) All events were performed in the presence or absence of Fc stimulation for 3 days. NKs were incubated on the surface to provide Fc sti...
Embodiment 2
[0117] IL-21 Up-regulates the Expression of Granzyme B in Human NK Cells
[0118] Human NK cells were isolated from Ficoll-Paque purified monocytes by negative selection using a Magnetic Bead Isolation Kit (Miltenyi Biotech, CA). Purified NK cells were then cultured in culture medium alone or in the presence of 20 ng / mL human IL-21. Cells are harvested, washed, and stained with surface markers. After surface marker staining, cells were washed and then treated with Cytofix / Cytoperm TM Cells were permeabilized with buffer (BD Biosciences, San Jose, CA) for 20 minutes. Cells were then stained with APC-labeled anti-human granzyme B antibody or an isotype control antibody (Caltag, Burlingame, CA) in Perm / Wash buffer. Wash the cells, then in the FACSCalibur TM Read on flow cytometer. Use Cellquest TM Software (BD Biosciences) was used to analyze the data.
[0119] figure 1 showed that incubation of human NKs in the presence of IL-21 resulted in a dramatic increase in the ...
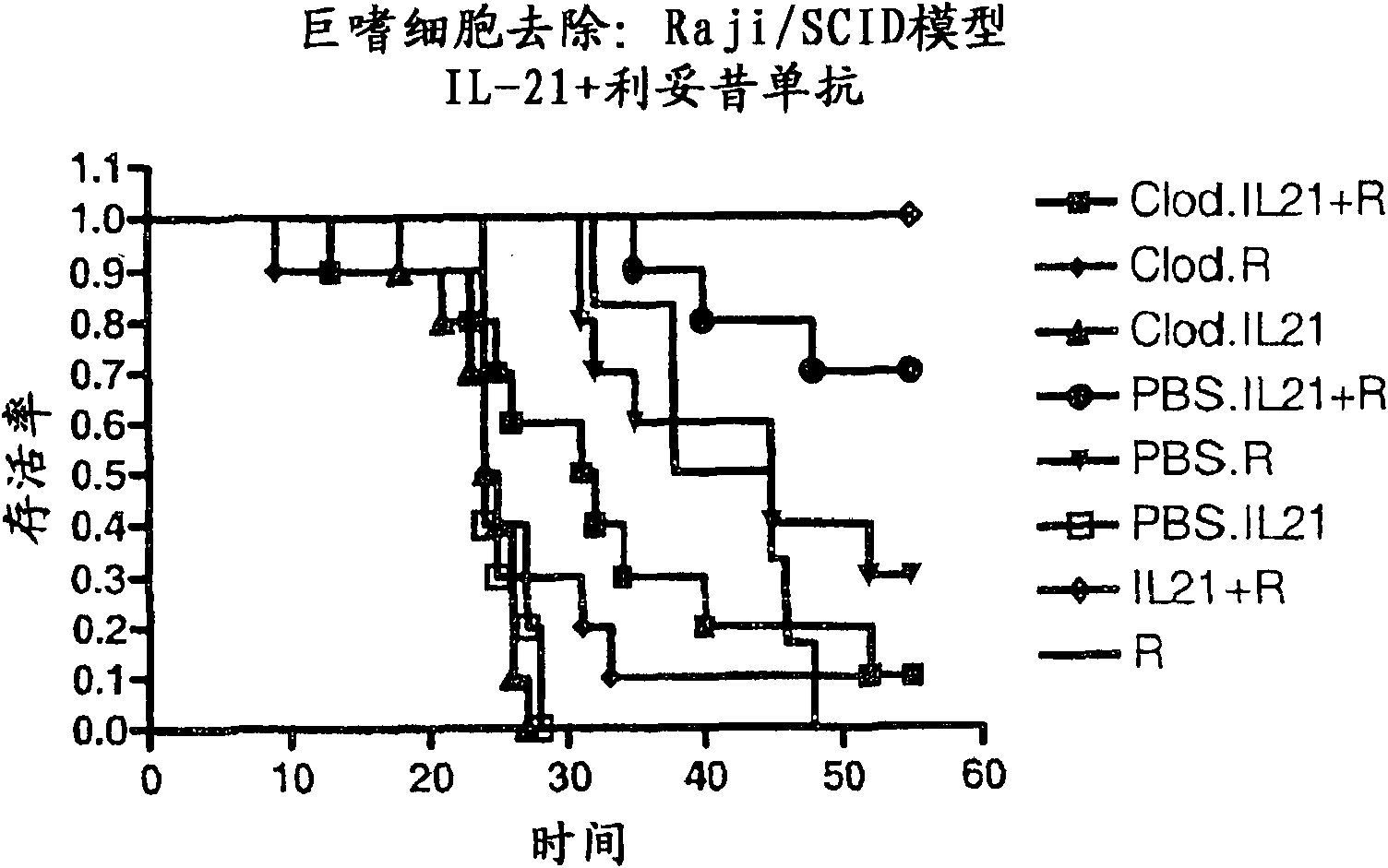
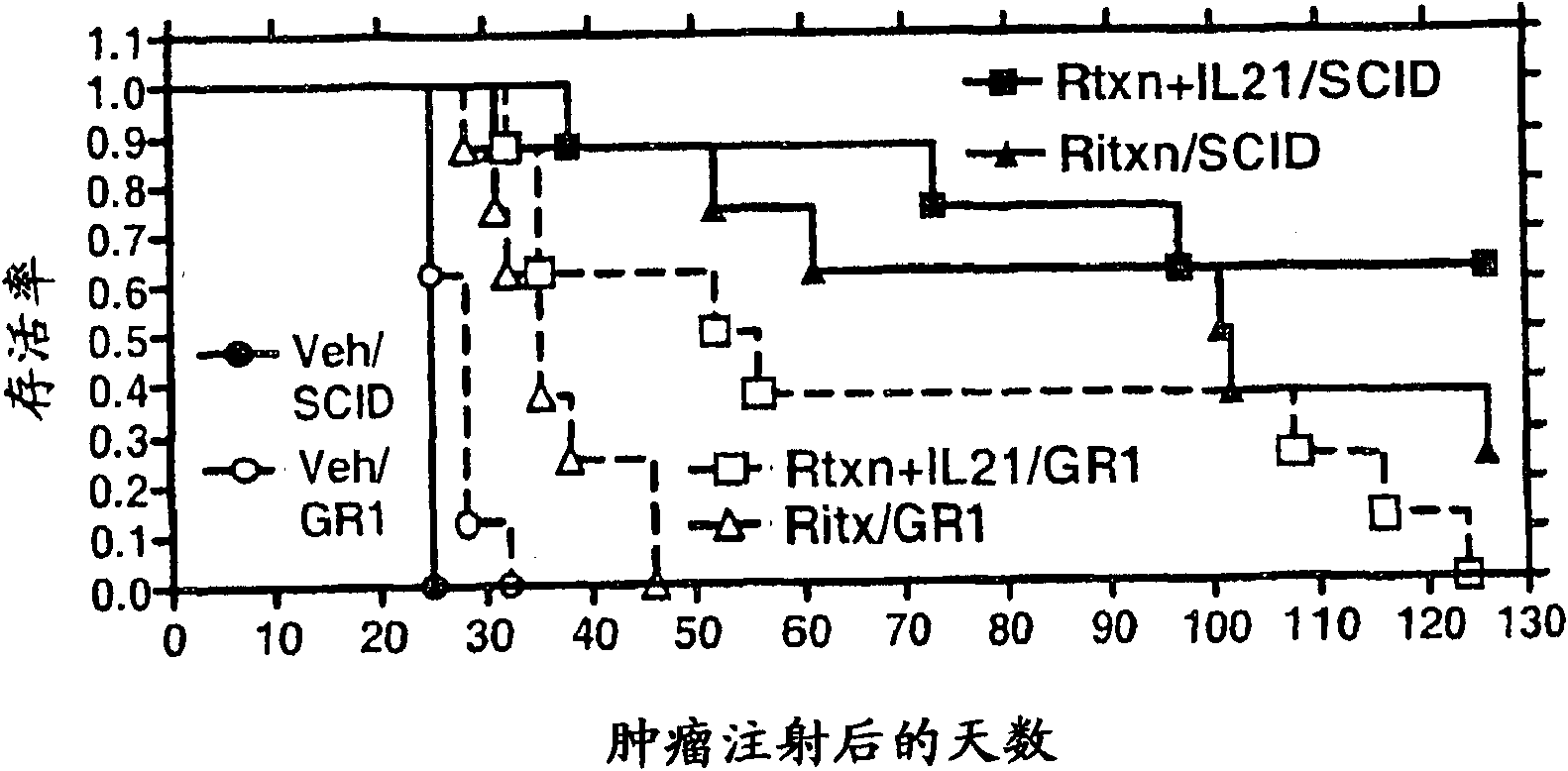
Embodiment 3
[0121] IL-21 + rituximab increased the expression of mice injected with HS Sultan lymphoma cells survival rate
[0122] A study was performed to assess whether tumor growth was delayed in CB-17 SCID mice injected with HS-Sultan cells, treated with rituximab, mouse IL-21 (mIL-21 ) or the combination of mIL-21 and rituximab treated mice. The study was designed to characterize the survival of HS-Sultan-loaded mice in each treatment group.
[0123] The protocol described and those known in the art (see, Cattan et al. Leuk Res. 18(7) : 513-522, 1994; Ozaki et al., Blood 90 (8) : 3179-86, 1997) similarly. CD17-SCID mice were administered 20 μg of rituximab (dosed every 4 days for a total of 5 injections), 100 μg of mIL-21 (dosed for 5 days) or rituximab by intraperitoneal route injection Combination of anti and mIL-21 (5 doses per treatment).
[0124] Mice were monitored for moribund or non-viable conditions such as paralysis or rapid weight loss. Body weights were co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com