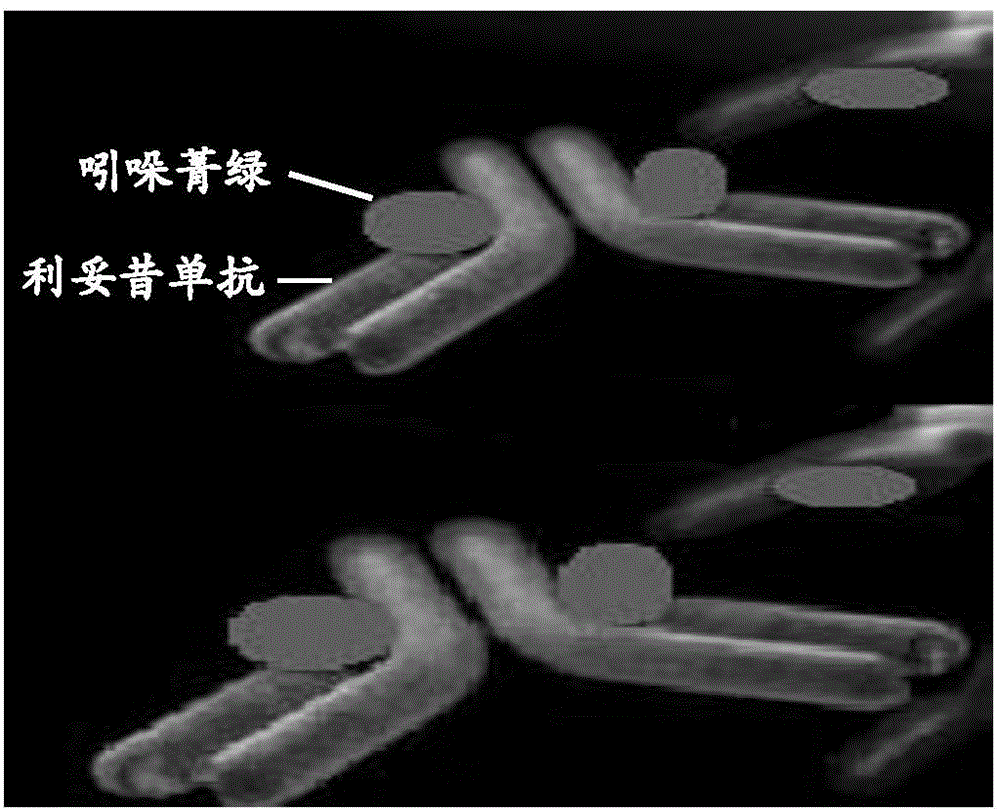
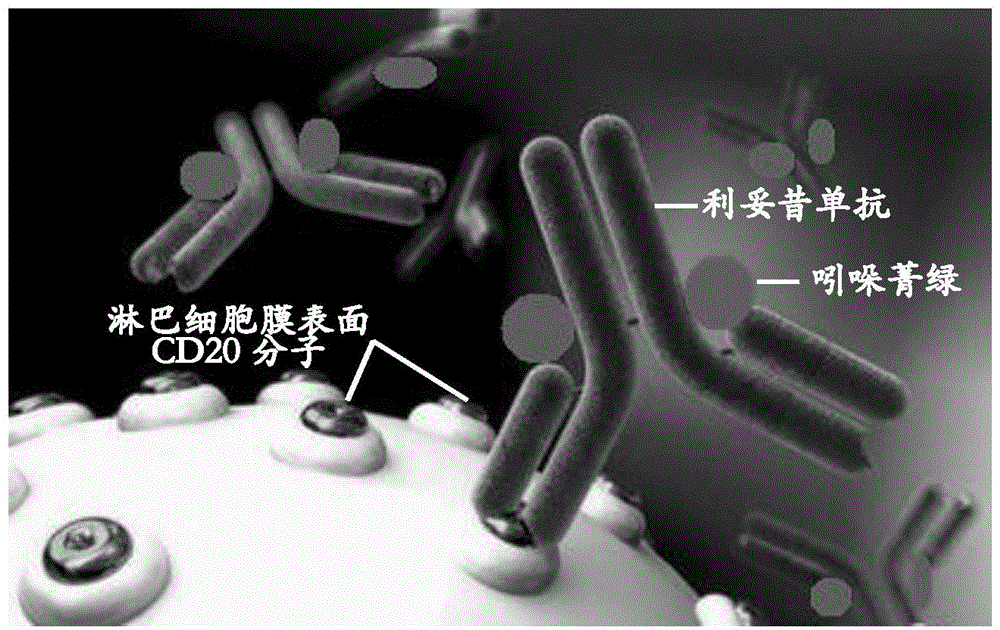
Application of indocyanine green-rituximab in preparation of breast cancer sentinel node tracer
A technology of rituximab and sentinel lymph node, which is applied in the field of specific tracers, can solve the problems of complex preparation process, radionuclide pollution, and inaccurate imaging, and achieve high sensitivity, difficulty in dissociation, and clear imaging. The effect of high image rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Application of indocyanine green-rituximab in preparation of sentinel lymph node tracer for breast cancer.
[0036] At room temperature, according to the principle of aseptic operation, 100mg of rituximab (Rituxan 100mg Roche) was made into 10mg / ml with 10ml of sterile water for injection (5ml of sterile water for injection, Tianjin Pharmaceutical Group Xinzheng Co., Ltd.) The solution is placed in a 50ml sterile centrifuge tube and stored under refrigeration (2-8°C) for later use; 25mg indocyanine green (indocyanine green for injection 25mg Dandong Medical Chuangye) is formulated with 10ml sterile water for injection to a concentration of The 2.5mg / ml solution should be stored in a 50ml sterile centrifuge tube and protected from light for later use. At room temperature, take 6 portions of 0.5ml of the prepared 10mg / ml rituximab solution and place them in a 10ml centrifuge tube, and then take the mass ratio of rituximab to indocyanine green to 3 : 1, 4:1, 6:1, 12:1, 30:1 ...
Embodiment 2
[0037] Example 2 Detecting the molecular integrity of the indocyanine green-rituximab coupling reactant:
[0038] 8% non-reducing SDS polyacrylamide gel electrophoresis was used to detect the molecular integrity of indocyanine green-rituximab, and rituximab was used as a positive control to determine indocyanine green-rituximab Molecular integrity, using the coupling reactants with mass ratios of rituximab and indocyanine green of 4:1, 6:1, 12:1, 30:1, and 32:1 in Example 1 did not appear Obviously degraded bands, such as Figure 4 As shown (the gel spectra of the coupling reactants in the above mass ratios are consistent, and no degradation bands are seen), it shows that the molecular structure of the coupling products of the two is complete.
Embodiment 3
[0039] Example 3 Detection of the immunological activity of the indocyanine green-rituximab conjugate:
[0040] Double antibody sandwich indirect enzyme-linked immunoassay (ELISA) method to detect the immune activity of indocyanine green-rituximab, rabbit anti-mouse IgG-Fab antibody is a coating antibody, and the mass fraction is 1% bovine serum albumin blocked For 1 hour, add the conjugates with mass ratios of rituximab to indocyanine green of 4:1, 6:1, 12:1, 30:1 and 32:1 in Example 1, and avoid at room temperature. Light reaction for 2 hours, add horseradish peroxidase-labeled goat anti-human IgG-Fc antibody, and react for 1 hour in the dark at room temperature. The color is developed by o-phenylenediamine hydrochloride. The enzyme-linked analyzer measures A 412nm Value such as Figure 5 As shown, the results show that the color reaction of the coupling reactants with mass ratios of rituximab and indocyanine green of 4:1, 6:1, 12:1, 30:1 and 32:1 was positive, and the OD 412 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com