Compound for Fab fragment of Rituximab and CD20 antigen epitope polypeptide
An antigen epitope, CD20 technology, applied in polycrystalline material growth, instrument, crystal growth, etc., can solve problems such as unclear mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, the preparation of antibody
[0022]Digest Rituximab (Roche) with papain, and the enzyme digestion conditions are: add papain (Sigma-Aldrich) to 10 mg / ml Rituximab to a final concentration of 0.1 mg / ml; then digest at 37°C for 18 hours, buffer Solution: 0.1M Tris-HCl+2mM EDTA+1mM Dithiothreitol, pH 8.0.
[0023] The digested product was first subjected to cation exchange chromatography using SP-Sepharose FF (GE Healthcare), and then subjected to hydrophobic interaction chromatography using Phenyl-Sepharose HP (GE Healthcare); through the above steps, the Fab fragment of Rituximab was obtained.
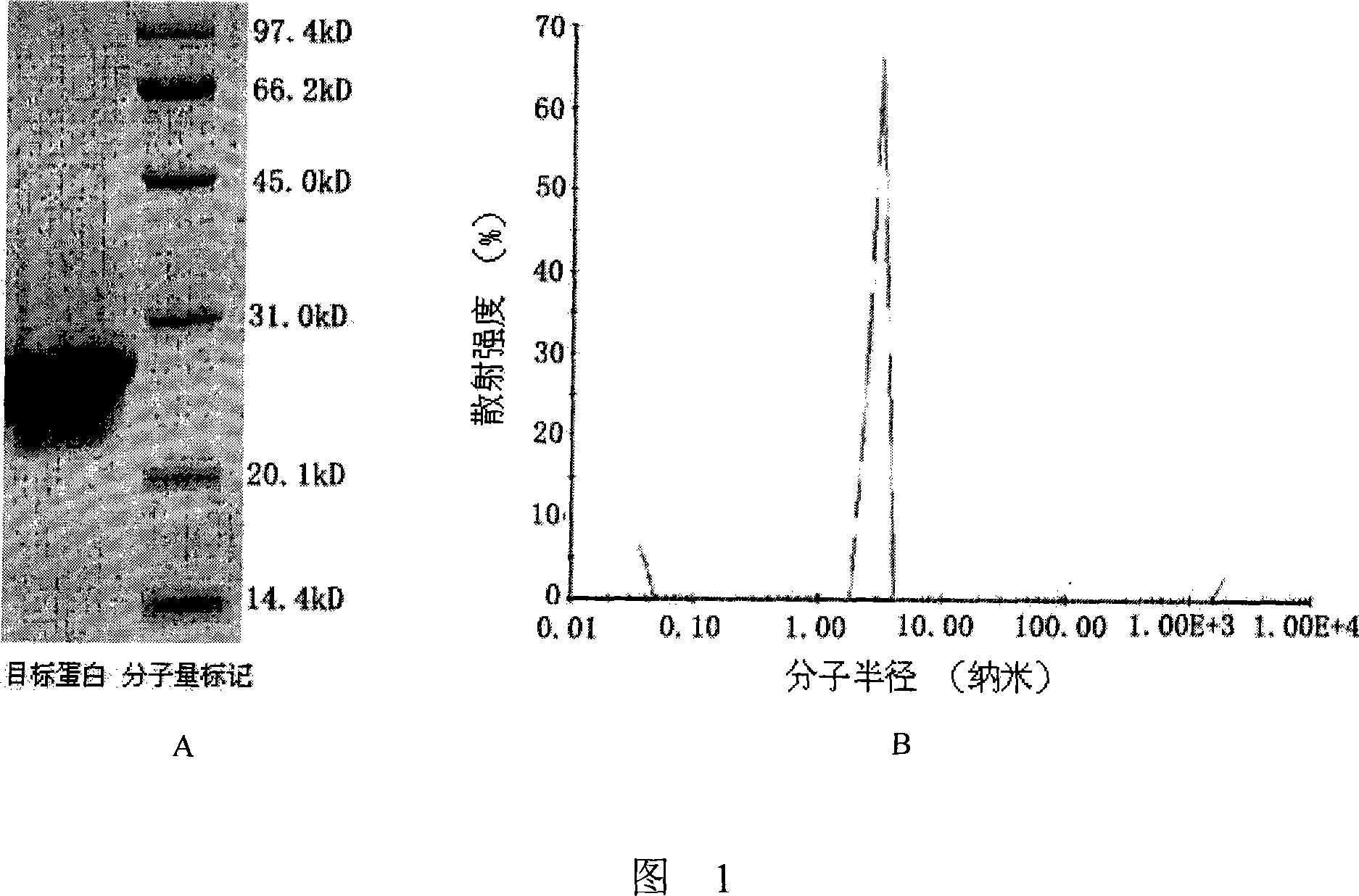
[0024] The purity and homogeneity of the Fab fragments were verified by SDS-PAGE and dynamic light scattering experiments, and the results are shown in Figure 1; sex is good.
[0025] The purified Fab fragment was concentrated to 8 mg / ml, and the buffer was replaced with: 100 mM NaCl+10 mM Tris-HCl, pH 8.0.
Embodiment 2
[0026] Embodiment 2, the preparation of CD20 epitope peptide
[0027] Corresponding to the peptide segment of residues 163 to 187 in the extracellular region of human CD20, an epitope peptide fragment containing 25 amino acids was designed and synthesized. The sequence of the fragment is as follows:
[0028] NIYN C EPANPSEKNSPSTQY C YSIQ.
[0029] At the same time, a chain was introduced between the two cysteines corresponding to residues 167 and 183 of the human CD20 extracellular region (the cysteines have been underlined in the sequence) in this fragment internal disulfide bonds to mimic the natural state of CD20.
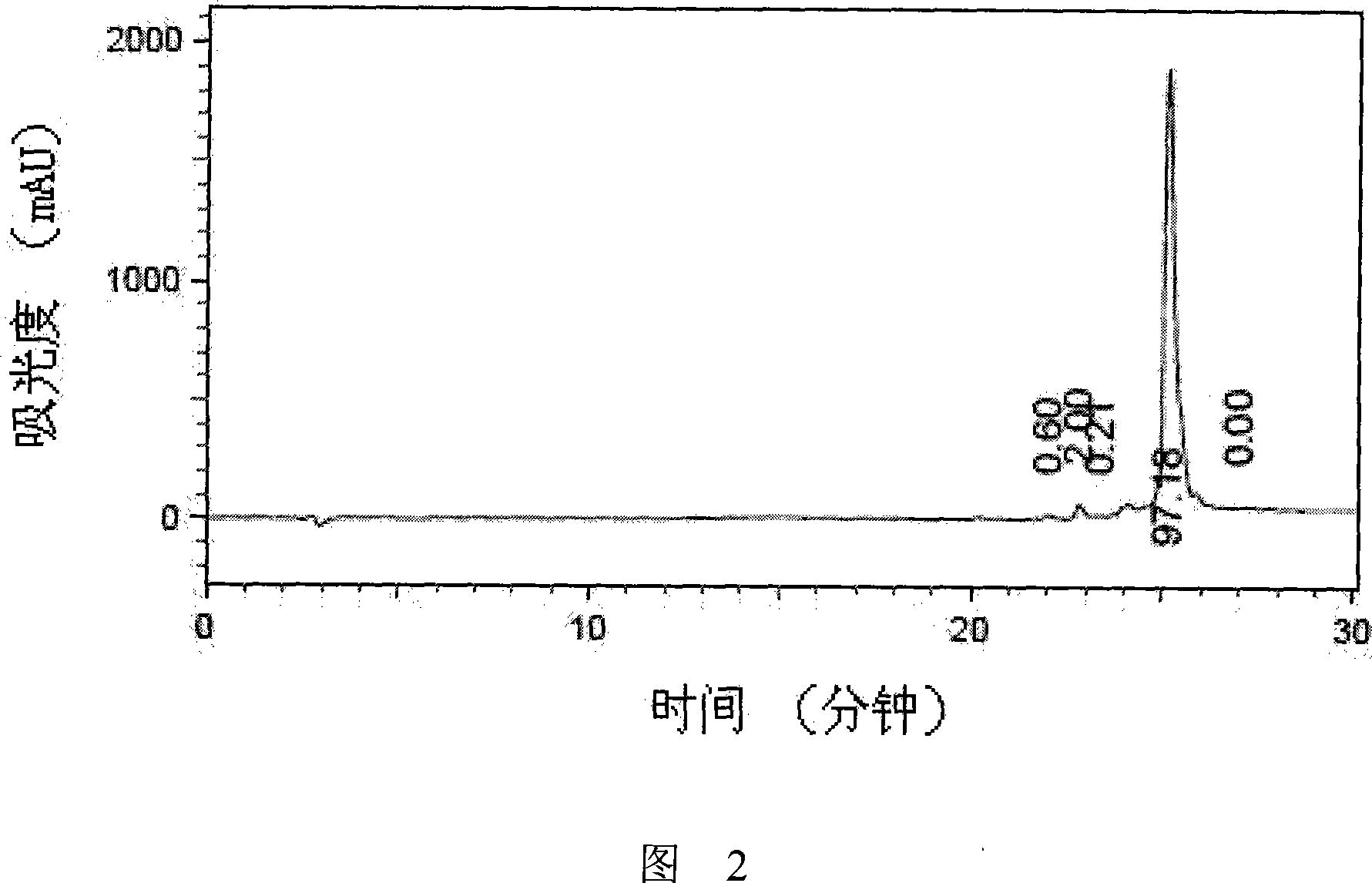
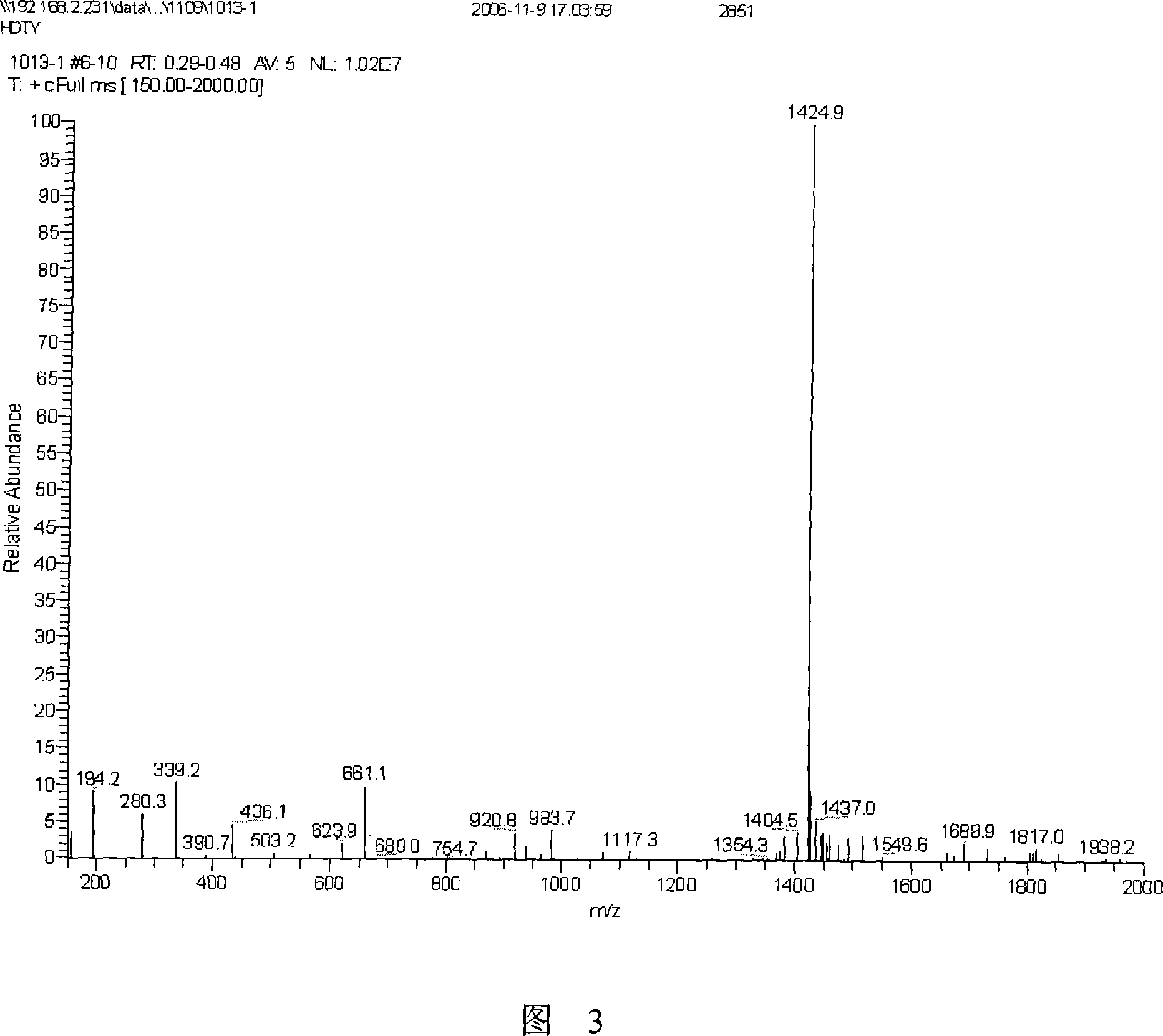
[0030] Referring to the method described in the operation manual, the purity of the prepared amino acid fragments was determined to be greater than 95% through analytical reversed-phase chromatography ( FIG. 2 ) and mass spectrometry ( FIG. 3 ).
Embodiment 3-5
[0031] Example 3-5, Co-crystallization of Antibody and Epitope Peptide
[0032] According to the molar ratio of 1:1, 1:5 and 1:10 respectively, the purified Rituximab Fab and CD20 epitope peptide were mixed at 4° C. for 12 hours to obtain a protein-peptide mixed solution.
[0033] Using the hanging drop diffusion method, mix the obtained protein-peptide mixed solution and the crystallization pool solution (0.2M calcium acetate, 0.1M sodium cacodylate, pH 6.5, 18% polyethylene glycol 8000) each 1 microliter After mixing to form hanging drops, place them in a crystallization tank containing 500 microliters of crystallization bath liquid for crystal growth.
[0034] After about two weeks of growth at 4°C, square-shaped co-crystallized complex crystals were harvested.
[0035] Crystals were stored frozen in Paratone-N (Hampton Research) and rapidly cooled to -170°C.
[0036] On the NW12 ray source (Japan Photon Factory), carry out the diffraction experiment of the co-crystal com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com