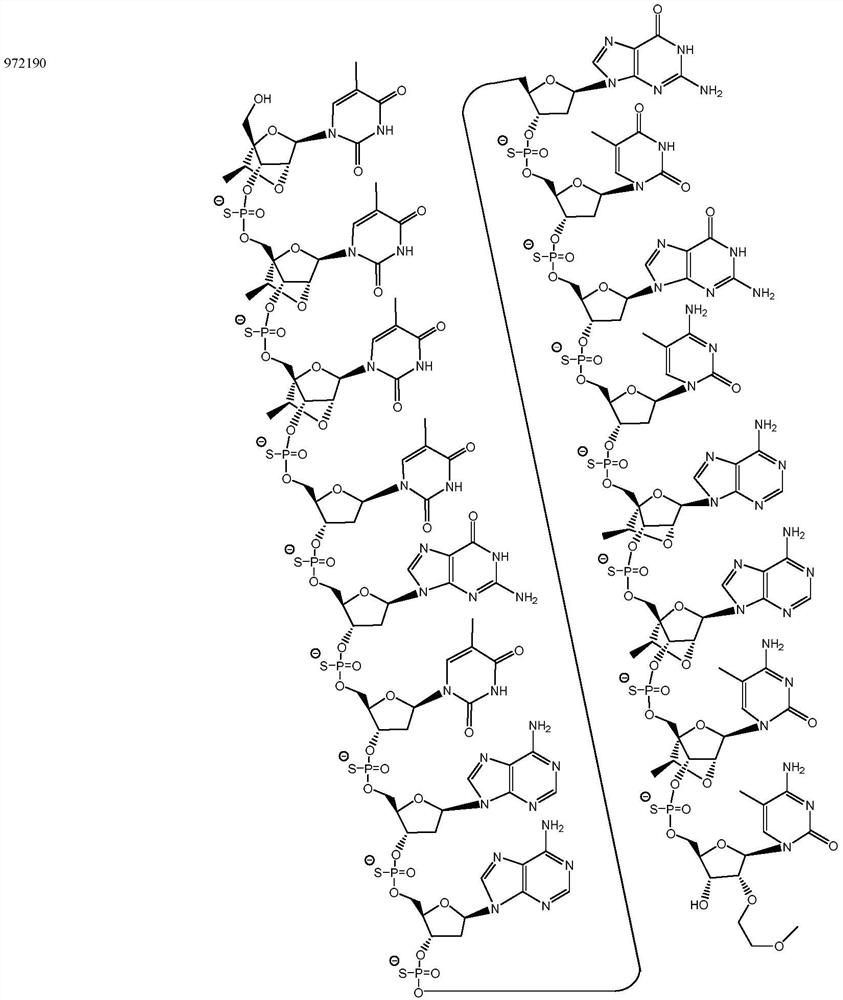
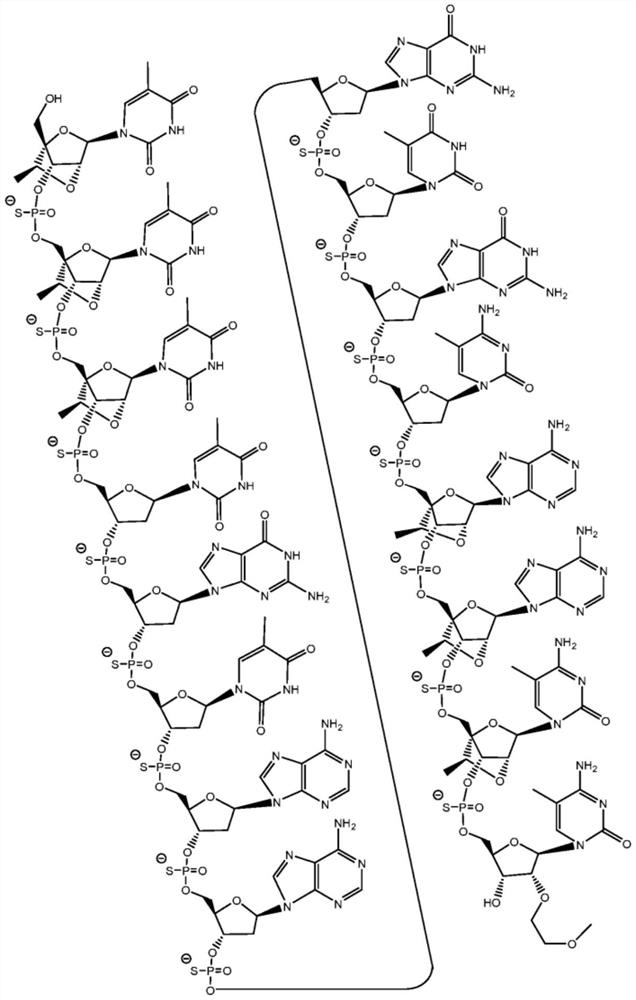
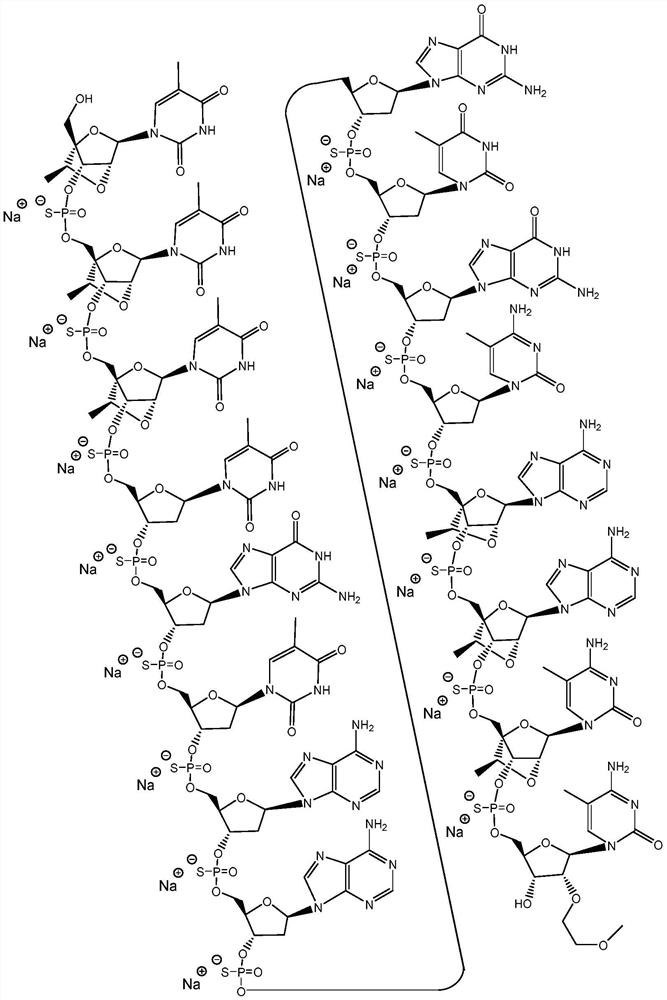
Modulators of apol1 expression
A technology of -O-2 and oligonucleotide, which is applied in the direction of recombinant DNA technology, DNA/RNA fragments, medical preparations containing active ingredients, etc., can solve the problem that the disease cannot be completely prevented from progressing, so as to slow down the progress and achieve high The effect of therapeutic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0356] The following example describes the screening process used to identify lead compounds targeting APOL1. For example, ION793406, 904763, 905469, 905505, 905634, 905665, 972190 and 972163 resulted in high potency and tolerability. ION 972190 exhibited high potency and tolerability.
[0357] Non-limiting disclosure and incorporation by reference
[0358]Although the sequence listing accompanying this document identifies each sequence as "RNA" or "DNA" as appropriate, in practice those sequences may be modified with any combination of chemical modifications. Those skilled in the art will readily recognize that terms such as "RNA" or "DNA" to describe modified oligonucleotides are in some cases arbitrary. For example, an oligonucleotide comprising a nucleoside containing a 2'-OH sugar moiety and a thymine base can be described as DNA with a modified sugar (2'-OH for the natural 2'-H of DNA) or as having RNA with modified bases (thymine (methylated uracil) for RNA's natural...
example 1
[0363] Example 1: Antisense inhibition of human APOL1 in A431 cells
[0364] Antisense oligonucleotides with various chemical motifs targeting APOL1 nucleic acids were designed and tested for their effect on APOL1 mRNA in vitro.
[0365] 3-10-3 cEt Notch body
[0366] The newly designed chimeric antisense oligonucleotides in the table below were designed as 3-10-3 cEt gapmers. These gap bodies are 16 nucleosides long, with a central gap segment consisting of ten 2'-deoxynucleosides and flanked in the 5' and 3' directions by wing segments each comprising three nucleosides . Every nucleoside in the 5' wing segment and every nucleoside in the 3' wing segment has a cEt modification. The internucleoside linkages throughout each notch body are phosphorothioate (P=S) linkages. All cytosine residues throughout each notch body are 5-methylcytosine.
[0367] "Start site" indicates the most 5' nucleoside in the human gene sequence to which the Notch body is targeted. "Stop site" ...
example 2
[0554] Example 2: Dose-dependent antisense inhibition of human APOL1 in A431 cells
[0555] Notchsomes from Example 1 that exhibited significant APOL1 mRNA suppression in vitro were selected and tested in A431 cells at different doses. These antisense oligonucleotides were tested in a series of experiments with similar culture conditions. The results of each experiment are presented in a separate table as shown below.
[0556] Cells were plated at a density of 10,000 cells per well and transfected with free uptake of 3-10-3cEt Notchsome at various concentrations as indicated in the table below. After a treatment period of approximately 16 hours, RNA was isolated from the cells and APOL1 mRNA levels were measured by quantitative real-time PCR. The human primer probe set RTS35962 was used to measure mRNA levels. as passed For measurements, APOL1 mRNA levels were adjusted for total RNA content. Results are presented as percent inhibition of APOL1 relative to untreated contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com