Lurasidone nanosuspension and preparation method thereof
A nano-suspension, lurasidone technology, applied in the directions of emulsion delivery, nervous system disease, liquid delivery, etc., can solve the problems of drop, poor water solubility, etc., and achieve simple process, increase solubility, and reduce particle size. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
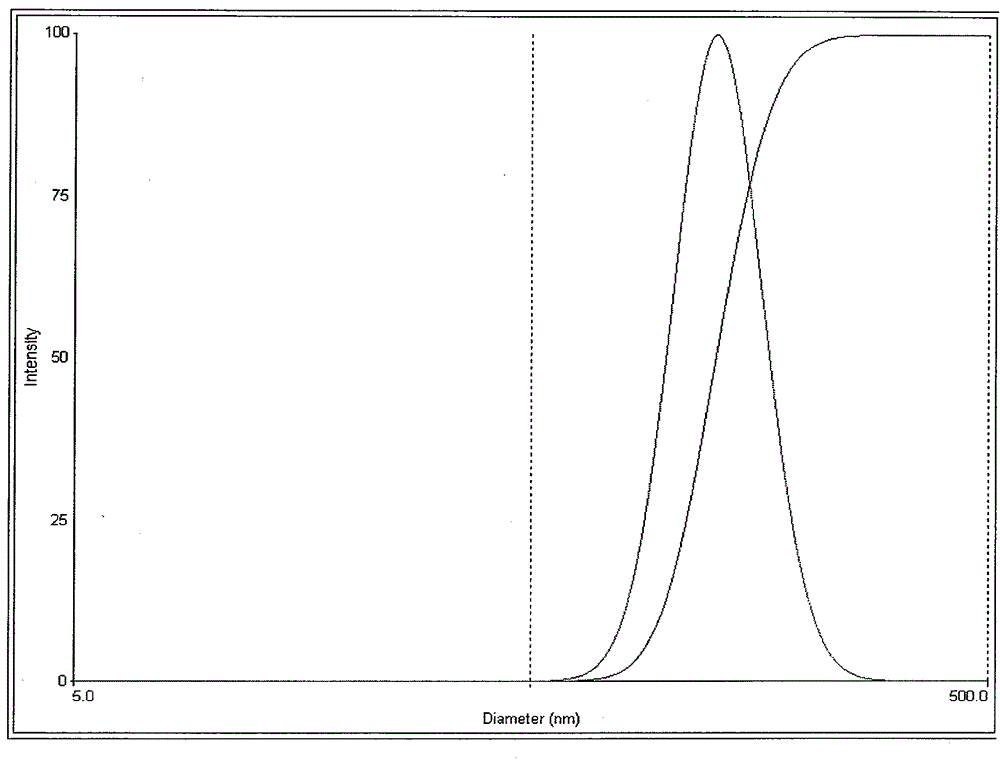
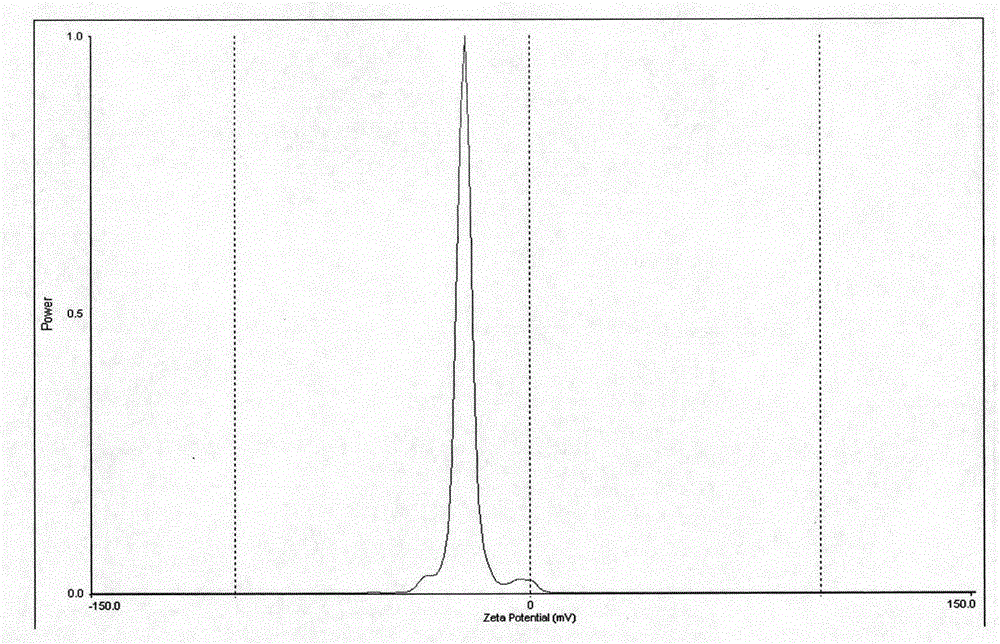
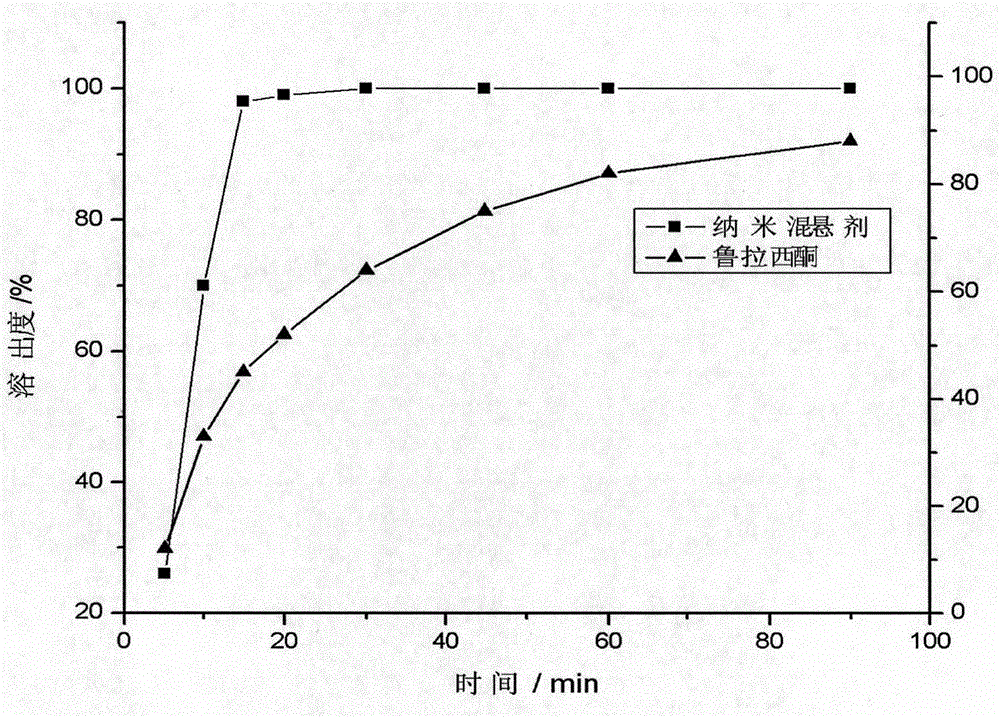
[0038] Put 40mg of lurasidone in a beaker, add 3mL of methanol, and ultrasonically dissolve it as the organic phase; then take 40mg of sodium lauryl sulfate, 20mg of poloxamer F68, and 20mg of polyvinylpyrrolidone K30 in another beaker, 20 mL of deionized water was added therein, stirred and dissolved by a magnetic stirrer as the water phase. Under the condition of ice-water bath, slowly inject the organic phase into the water phase, and at the same time, use a high-speed disperser to disperse at a speed of 400r / min for 5 minutes to obtain the initial suspension, and circulate the initial suspension with a pressure of 1500bar and high-pressure homogeneity for 10 The second time, the organic solvent was removed by vacuum drying at 25°C for 24 hours. The particle size was measured by a Brookhaven Instruments-ZetaPlus nanometer particle size analyzer to be 159nm, the polydispersity coefficient was 0.082, and the zeta potential was -24.8mv.
[0039] Add 2.5 g of mannitol to the pr...
Embodiment 2
[0041] Put 40mg of lurasidone in a beaker, add 3mL of methanol, and ultrasonically dissolve it as the organic phase; then take 40mg of sodium lauryl sulfate, 20mg of poloxamer F68, and 20mg of polyvinylpyrrolidone K30 in another beaker, 20 mL of deionized water was added therein, stirred and dissolved by a magnetic stirrer as the water phase. Under the condition of ice-water bath, slowly inject the organic phase into the water phase, and at the same time, use a high-speed disperser to disperse at a speed of 400r / min for 5 minutes to obtain the initial suspension, and circulate the initial suspension with a pressure of 1500bar and high-pressure homogeneity for 10 The second time, the organic solvent was removed by vacuum drying at 25°C for 24 hours. The particle size was measured by a Brookhaven Instruments-ZetaPlus nanometer particle size analyzer to be 136nm, the polydispersity coefficient was 0.142, and the zeta potential was -22.8mv.
Embodiment 3
[0043] Take 40 mg of lurasidone and place it in a beaker, add 3 mL of methanol, and ultrasonically dissolve it as the organic phase; then take 40 mg of sodium lauryl sulfate, 10 mg of poloxamer F68, and 5 mg of polyvinylpyrrolidone K30 in another beaker, 20 mL of deionized water was added therein, stirred and dissolved by a magnetic stirrer as the water phase. Under the condition of ice-water bath, slowly inject the organic phase into the water phase, and at the same time, use a high-speed disperser to disperse at a speed of 400r / min for 5 minutes to obtain the initial suspension, and circulate the initial suspension with a pressure of 1500bar and high-pressure homogeneity for 10 The second time, the organic solvent was removed by vacuum drying at 25°C for 24 hours. The particle size was measured by Brookhaven Instruments-ZetaPlus nanometer particle size analyzer to be 281nm, the polydispersity coefficient was 0.054, and the zeta potential was -26.4mv.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com