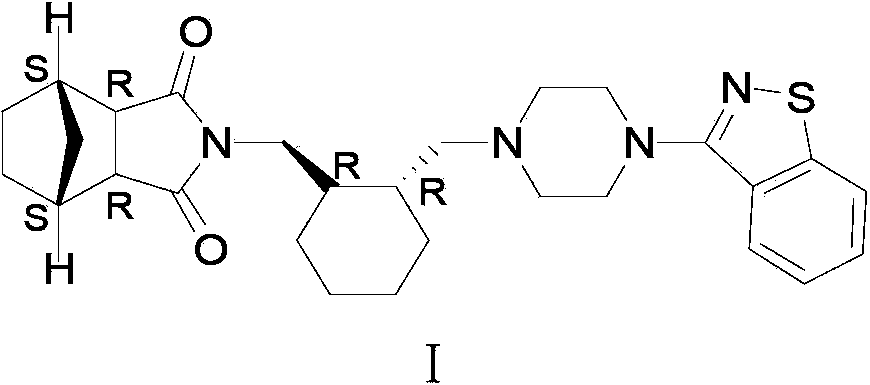
Method for preparing lurasidone
A technology of lurasidone and benzo, which is applied in the field of preparation of lurasidone, can solve the problems of complex process operation and low product yield, and achieve cheap and easy reagents, high total yield and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
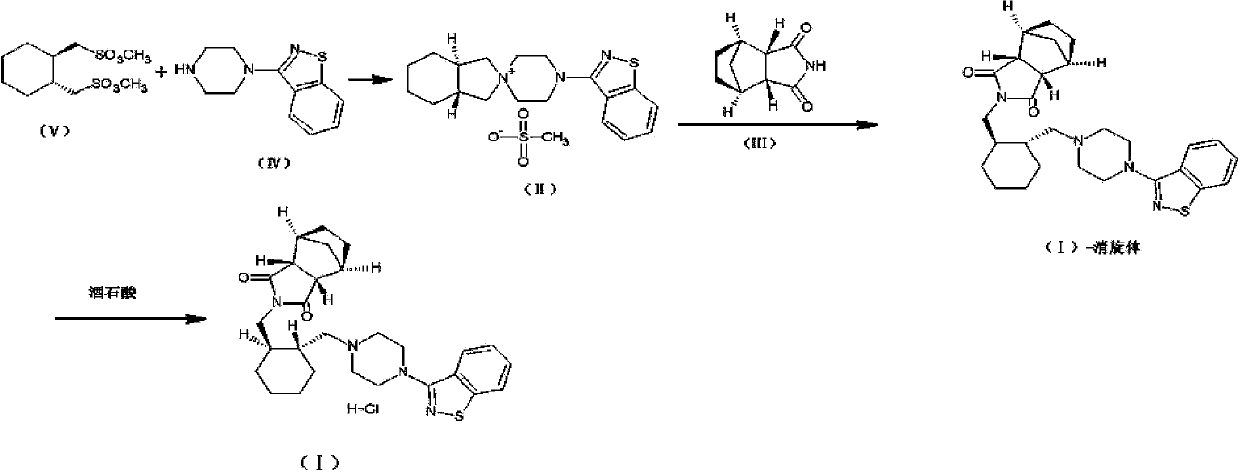
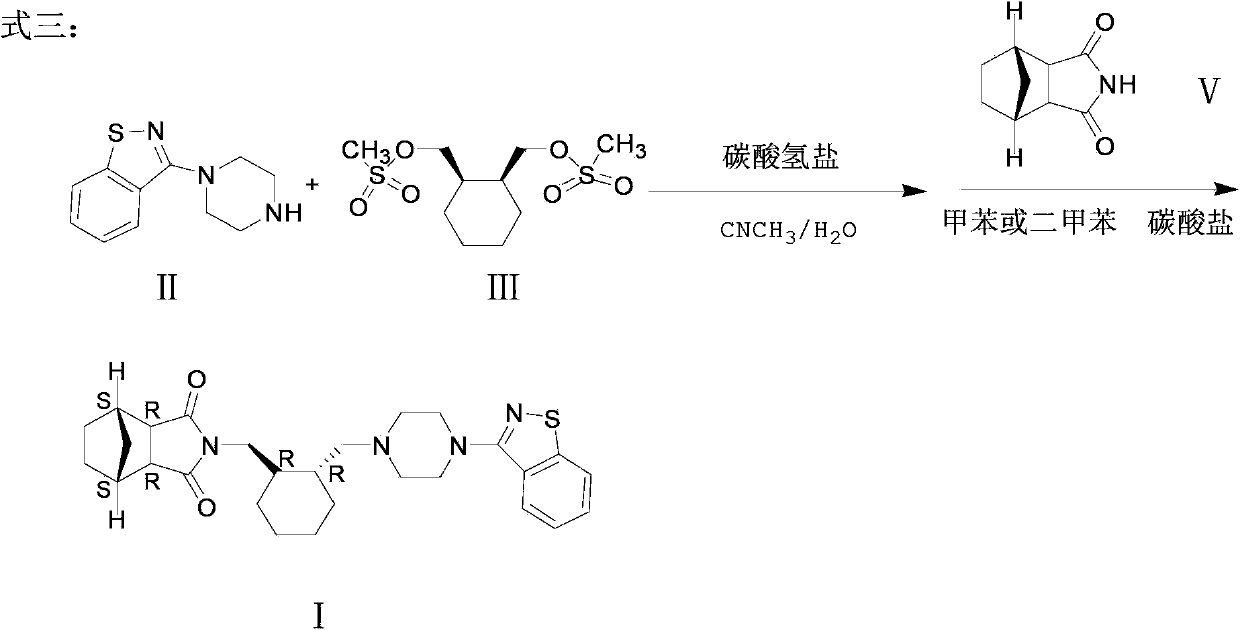
[0041] Add (R,R)-1,2-bis(methylsulfonyl-2-oxymethyl)cyclohexane (10.0 g, 0.033 mol) and 1-(1,2-benzisothiazole-3 - Base) piperazine (7.7g, 0.033mol), then add 100ml of acetonitrile, 7.0g (0.066mol) of sodium carbonate, stir and reflux for 24 hours, TLC (methanol:petroleum ether=8:1) detects that there is still raw material 1- (1,2-benzisothiazol-3-yl)piperazine did not complete the reaction, continue to react for 24 hours, TLC (methanol:petroleum ether=8:1) detection raw material point 1-(1,2-benzisothiazole-3 - Base)piperazine had a little unreacted, the solvent was spin-dried, slurried with 50ml of toluene at 100°C for 2 hours, filtered hot, and the solid was dried to obtain 7.12g of the product, with a yield of 51.0%.
[0042] Add 7.12g of the product from the previous step and 2.78g (0.0168mol) of bicyclo[2.2.1]heptane-2,3-dicarboximide (Compound V) into the reaction flask, add 70ml of solvent toluene, 5.8g of potassium carbonate ( 0.042mol), heated and refluxed for 12 ho...
Embodiment 2
[0044]Add (R,R)-1,2-bis(methylsulfonyl-2-oxymethyl)cyclohexane (10 g, 0.033 mol) and 1-(1,2-benzisothiazole-3- Base) piperazine (7.7g, 0.033mol), then add 50ml of acetonitrile, sodium carbonate 9.5g (0.090mol), stir and reflux for 48 hours, TLC (methanol:petroleum ether=8:1) detection of raw materials 1-(1, 2 benzisothiazol-3-yl)piperazine was not completely reacted, and the reaction was continued for 8 hours, and then bicyclo[2.2.1]heptane-2,3-dicarboximide (compound V) (5.45g. 0.033mol) and toluene 100ml, continue to reflux for 12 hours, TLC (ethyl acetate:petroleum ether=1:1) detects that compound IV has not reacted completely, the target product lurasidone is rarely formed, and there are many impurities, spin-dried organic solvent, recrystallization from ethyl acetate failed to yield a solid.
Embodiment 3
[0046] Add (R,R)-1,2-bis(methylsulfonyl-2-oxymethyl)cyclohexane (10 g, 0.033 mol) and 1-(1,2-benzisothiazole-3- Base) piperazine (7.7g, 0.033mol), then add 100ml acetonitrile, sodium carbonate 9.5g (0.090mol), stir and reflux for 48 hours, TLC (methanol:petroleum ether=8:1) detection raw material 1-(1, 2 benzisothiazol-3-yl)piperazine was not completely reacted, and the reaction was continued for 8 hours, and then bicyclo[2.2.1]heptane-2,3-dicarboximide (compound V) (5.45g. 0.033mol), continue to reflux for 12 hours, TLC (ethyl acetate:petroleum ether=1:1) detects that compound Ⅳ has not reacted completely, the target product lurasidone is less generated, and there are more impurities. Recrystallization from ethyl ester failed to yield a solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com