Preparation method of chiral intermediate cyclohexane dimethanol
A technology of cyclohexanedimethanol and chiral intermediate, applied in the field of medicine, can solve the problems of high cost, large amount of red aluminum, unfavorable industrial production, etc., and achieves the effects of low price and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
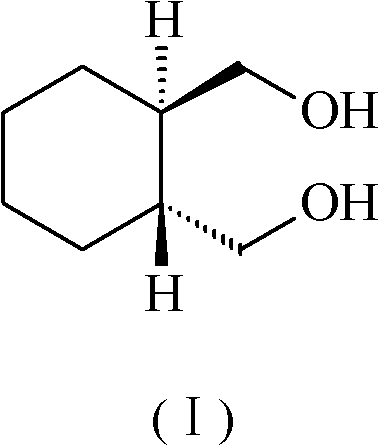
[0014] Dissolve 17.2 g (0.1 mol) of 1R, 2R-cyclohexanedicarboxylic acid in 340 ml of tetrahydrofuran, add 3.8 g (0.1 mol) of sodium borohydride, and 14.5 g (0.1 mol) of boron trifluoride diethyl ether (content 47%) , slowly heated to 70°C for reflux reaction for 3 hours, recovered tetrahydrofuran under reduced pressure, evaporated to dryness, added dropwise aqueous sodium hydroxide solution, adjusted pH = 11, filtered, extracted the filtrate with ethyl acetate three times, combined the ethyl ester solution, and washed with brine , dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness to obtain 14 g of 1R, 2R-cyclohexanedimethanol.
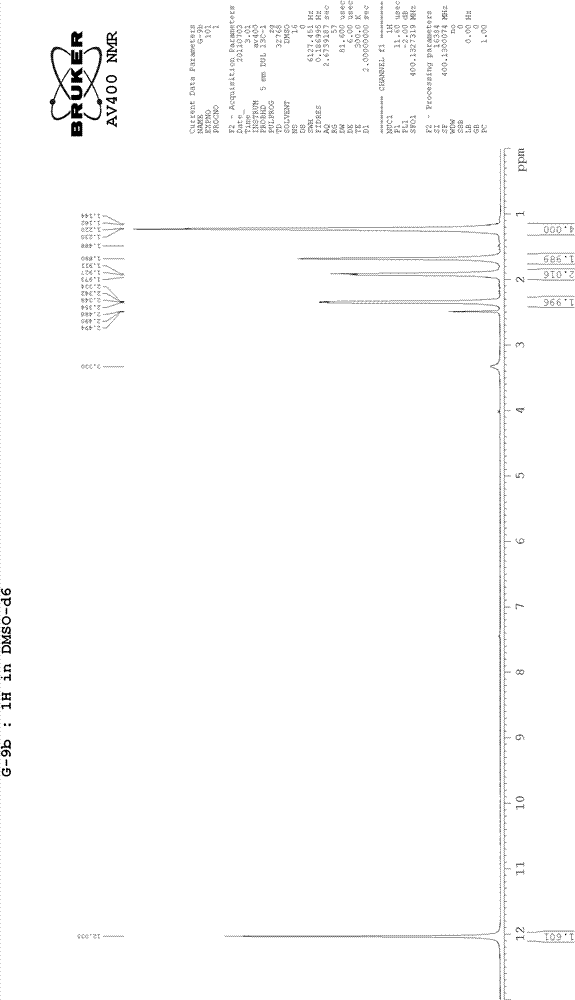
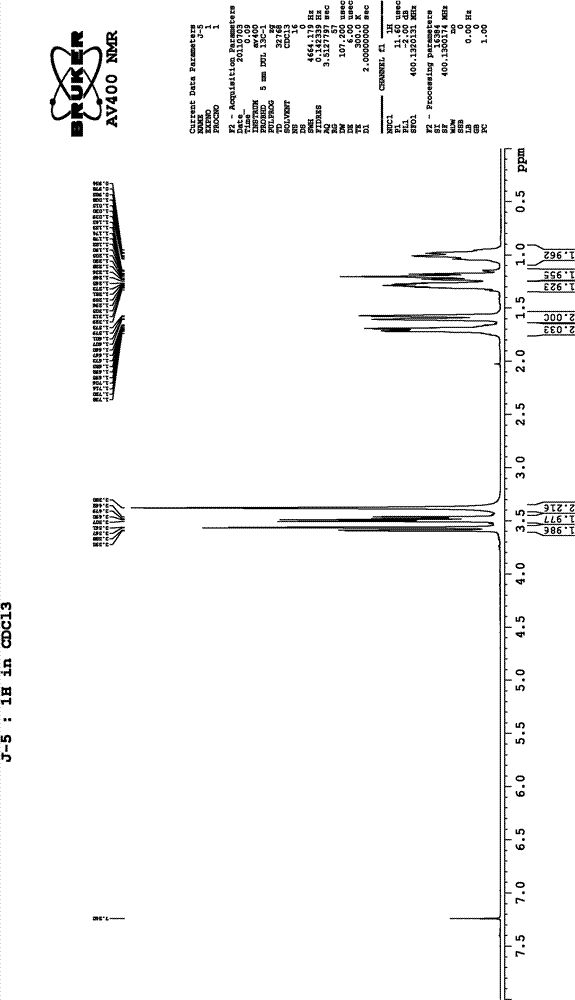
[0015] 1 H-NMR (CDCl 3 )δ: 0.95-1.32 (m, 6H), 1.57-1.74 (m, 4H), 3.38-3.59 (m, 6H). (See figure 2 )
Embodiment 2
[0017] Dissolve 17.2 g (0.1 mol) of 1R, 2R-cyclohexanedicarboxylic acid in 680 ml of ether, add 15.2 g (0.4 mol) of sodium borohydride, and 145 g (1 mol) of boron trifluoride ether (content 47%), at room temperature React for 10 hours, recover tetrahydrofuran under reduced pressure, evaporate to dryness, add aqueous sodium hydroxide solution dropwise, adjust pH=11, filter, and extract the filtrate with ethyl acetate three times, combine the ethyl acetate solution, wash with brine, and dry over anhydrous magnesium sulfate. Filter and evaporate to dryness to obtain 13.5 g of 1R, 2R-cyclohexanedimethanol.
[0018] 1 H-NMR (CDCl 3 )δ: 0.95-1.32 (m, 6H), 1.57-1.74 (m, 4H), 3.38-3.59 (m, 6H). (See figure 2 )
Embodiment 3
[0020] 17.2 g (0.1 mol) of 1R, 2R-cyclohexanedicarboxylic acid was dissolved in 340 ml of tetrahydrofuran, and 54 g (1 mol) of potassium borohydride and 72.5 g (0.5 mol) of boron trifluoride diethyl ether (content 47%) were added successively, keeping React at 10°C for 15 hours, recover tetrahydrofuran under reduced pressure, evaporate to dryness, add aqueous sodium hydroxide solution dropwise, adjust pH=11, filter, extract the filtrate with ethyl acetate three times, combine the ethyl acetate solution, wash with brine, and anhydrous magnesium sulfate Dry, filter, and evaporate to dryness to obtain 13.5 g of 1R, 2R-cyclohexanedimethanol.
[0021] 1 H-NMR (CDCl 3 )δ: 0.95-1.32 (m, 6H), 1.57-1.74 (m, 4H), 3.38-3.59 (m, 6H). (See figure 2 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com