Lurasidone orally disintegrating tablet
A technology of orally disintegrating tablets and lurasidone, which is applied in the field of orally disintegrating tablets containing lurasidone and its salts and its preparation, can solve the problems of shortening the validity period of products, decreasing dissolution rate, unfavorable long-term storage of products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] lurasidone hydrochloride 20mg lactose 35mg silica 12.2mg microcrystalline cellulose 20mg Croscarmellose Sodium 10mg Menthol 0.3mg Sucralose 1.5mg Magnesium stearate 1mg Total 100mg
Embodiment 2
[0023] lurasidone hydrochloride 40mg Sorbitol 60mg silica 20mg microcrystalline cellulose 20mg Crospovidone 10mg Low-substituted hydroxypropyl cellulose 7mg mint flavor 1mg Acesulfame K 1mg Magnesium stearate 1mg Total 160mg
Embodiment 3
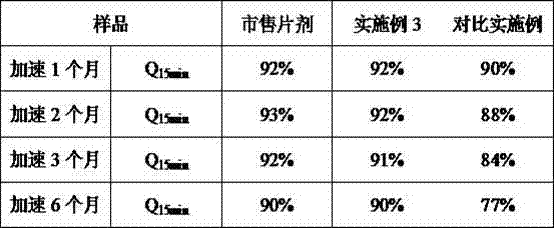
[0025] components Example 3 comparative example lurasidone hydrochloride 80mg 80mg Mannitol 80mg 115mg silica 35mg - microcrystalline cellulose 60mg 60mg Crospovidone 32mg 32mg Croscarmellose Sodium 20mg 20mg mint flavor 6.6mg 6.6mg Aspartame 3.2mg 3.2mg Magnesium stearate 3.2mg 3.2mg Total 320mg 320mg
[0026] (a) Preparation method:
[0027] 1. Co-powder lurasidone hydrochloride and mannitol to a particle size of 1-10 μm.
[0028] 2. Mix the above co-powder with silicon dioxide, microcrystalline cellulose and crospovidone evenly.
[0029] 3. Add 30% ethanol solution to the above mixture to make a soft material, pass through a 20-mesh sieve and granulate.
[0030] 4. Dry the prepared granules until the moisture L.O.D<3%, sizing the granules, adding additional auxiliary materials and mixing evenly after converting the yield.
[0031] 5. Measure the content of the main d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com