Lurasidone pharmaceutical composition and preparation method thereof
A technology of lurasidone and lurasidone hydrochloride is applied in the field of preparation compositions and preparations of antipsychotic drugs, and can solve patient injury, organic solvent types, dosage or content restrictions, hidden dangers in production safety, etc. problems, to avoid waste of raw materials, eliminate grinding and sieving, and improve production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] composition:
[0048] Lurasidone Hydrochloride 40 g
[0049] Hydroxypropyl-beta-cyclodextrin 86 g
[0050] Lactose 27.5 g
[0051] Croscarmellose Sodium 3.5 g
[0052] Hypromellose 2 g
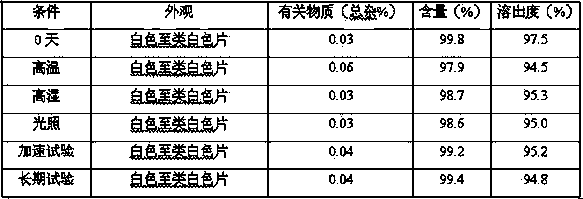
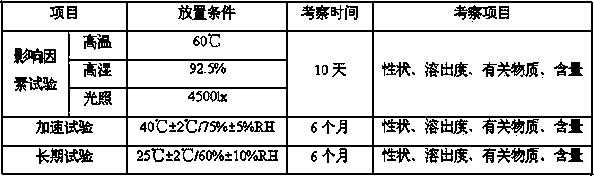
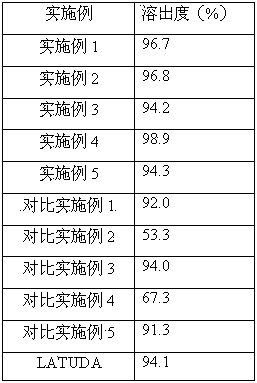
[0054] Preparation method: Add hydroxypropyl-β-cyclodextrin to water and stir to dissolve, add lurasidone hydrochloride to ethanol to dissolve, add the ethanol solution to the aqueous solution, continue to stir until the solution is clear, and spray dry to obtain lurasidone Ketocyclodextrin inclusion complex. Add lurasidone inclusion compound, lactose, and croscarmellose sodium into the fluidized bed, mix well, and then add hypromellose to granulate. Add magnesium stearate, mix well, and press into 1000 tablets.
[0055]
Embodiment 2
[0057] composition:
[0058] Lurasidone Hydrochloride 40 g
[0059] Hydroxypropyl-beta-cyclodextrin 114 g
[0060] Lactose 28.5 g
[0061] Sodium carboxymethyl starch 8 g
[0062] Hypromellose 8 g
[0064] Preparation method: Add hydroxypropyl-β-cyclodextrin to water and stir to dissolve, add lurasidone hydrochloride to ethanol to dissolve, add the ethanol solution to the aqueous solution, continue to stir until the solution is clear, and spray dry to obtain lurasidone Ketocyclodextrin inclusion complex. Add lurasidone inclusion complex, lactose, and sodium carboxymethyl starch into the fluidized bed, mix well, and then add hypromellose to granulate. Add magnesium stearate, mix well, and press into 1000 tablets.
[0065]
Embodiment 3
[0067] composition:
[0068] Lurasidone Hydrochloride 40 g
[0069] β-cyclodextrin 128 g
[0070] Mannitol 22.5 g
[0071] Sodium carboxymethyl starch 4 g
[0072] povidone 4 g
[0073] Magnesium stearate 1.5 g
[0074] Preparation method: add β-cyclodextrin to water and stir to dissolve, add lurasidone hydrochloride to ethanol to dissolve, add the ethanol solution to the aqueous solution, continue to stir until the solution is clear, and spray dry to obtain lurasidone cyclodextrin clathrate. Add lurasidone clathrate, mannitol and sodium carboxymethyl starch into the fluidized bed, mix well, then add povidone to granulate. Add magnesium stearate, mix well, and press into 1000 tablets.
[0075]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com