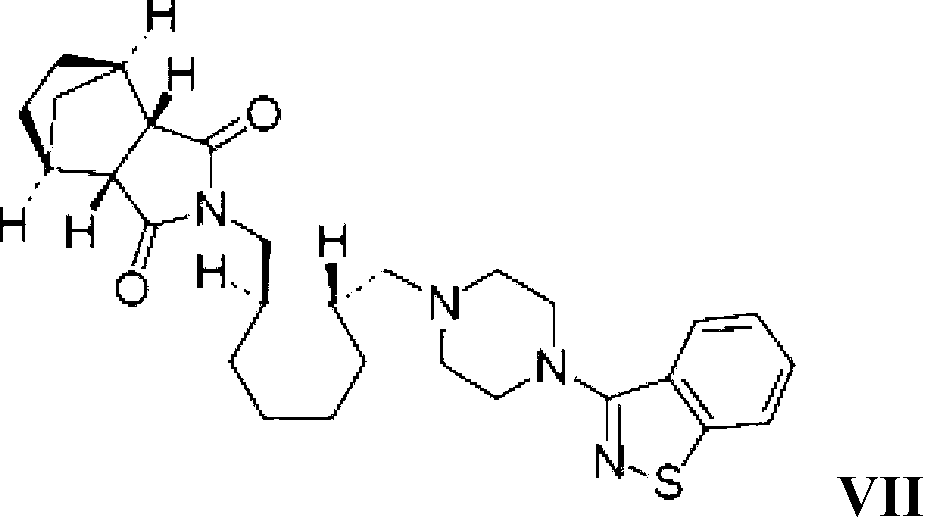
Method for preparing lurasidone
A compound and equivalent technology, applied in the field of atypical antipsychotic compounds, can solve the problems of low yield, high and low production cost of lurasidone, which generally can only reach about 35-42%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the preparation of the iodide of formula II intermediate
[0084]
[0085] In dichloromethane (DCM), add 1 equivalent of the compound of formula I at one time, triphenylphosphine (PPh 3 ) 1.2 equivalents, stirring in the greenhouse, adding elemental iodine (I 2 ) 1.2 equivalents, TLC chromogenic method monitors reaction, and chromogenic agent is phosphomolybdic acid chromogenic agent, to raw material formula I compound reaction completes, and column chromatography separates, and obtains the formula II intermediate compound containing iodine functional group, and this intermediate compound is White powder. The yield is about 95%.
Embodiment 2
[0086] Embodiment 2: the preparation of the iodide of formula II intermediate
[0087]
[0088] Add 1 equivalent of the compound of formula I and 2.2 equivalents of triethylamine (TEA) to dichloromethane (DCM) at one time, control the temperature below 0°C, add 2.2 equivalents of methanesulfonyl chloride (MsCl), and monitor the reaction by TLC chromogenic method. The chromogenic agent is phosphomolybdic acid chromogenic agent, until the reaction of the raw material compound I is completed, water is added, dichloromethane (DCM) is extracted, a solid is obtained after concentration, and 2.5 equivalents of sodium iodide (NaI) is added to the solid, an appropriate amount of Acetone (Acetone) was dissolved, reacted in the dark for 10 hours, added water to remove the solid, filtered to obtain the intermediate compound of formula II containing iodine functional group, the intermediate compound was a white powder, and the yield was about 87%.
Embodiment 3
[0089] Embodiment 3: the preparation of the bromide of formula II intermediate
[0090]
[0091] Using acetic acid (AcOH) as a solvent, add 1 equivalent of the compound of formula I, 5-10 equivalents of 40% hydrobromic acid (HBr), 0.1 equivalent of sulfuric acid, react at 60-80 °C, monitor the reaction by TLC color development, and the color developer is phosphorus Molybdic acid chromogenic agent, until the reaction of the raw material formula I compound is completed, add water, dichloromethane (DCM) extraction, after concentration, obtain the formula II intermediate compound containing bromine functional group, this compound is an oil, and the yield is about 92- 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com