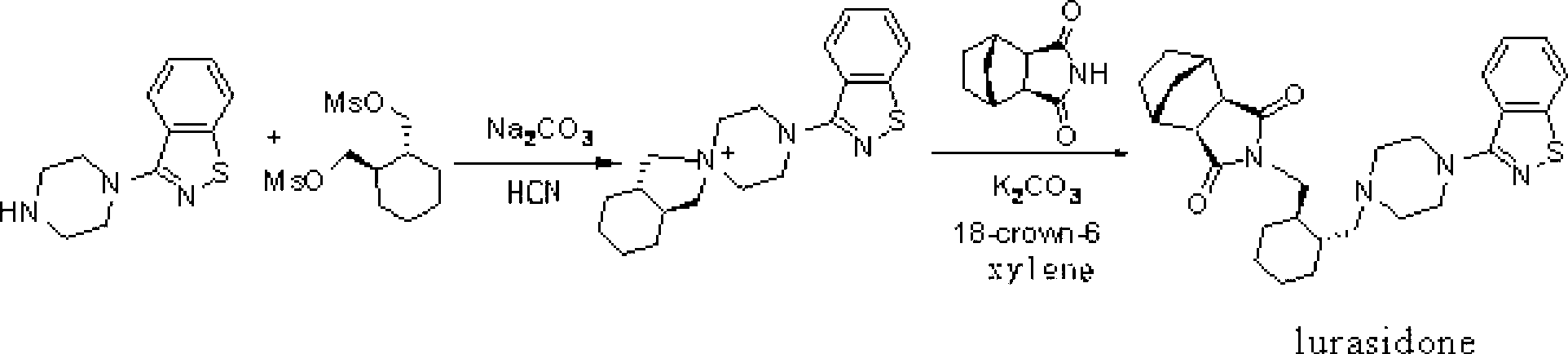
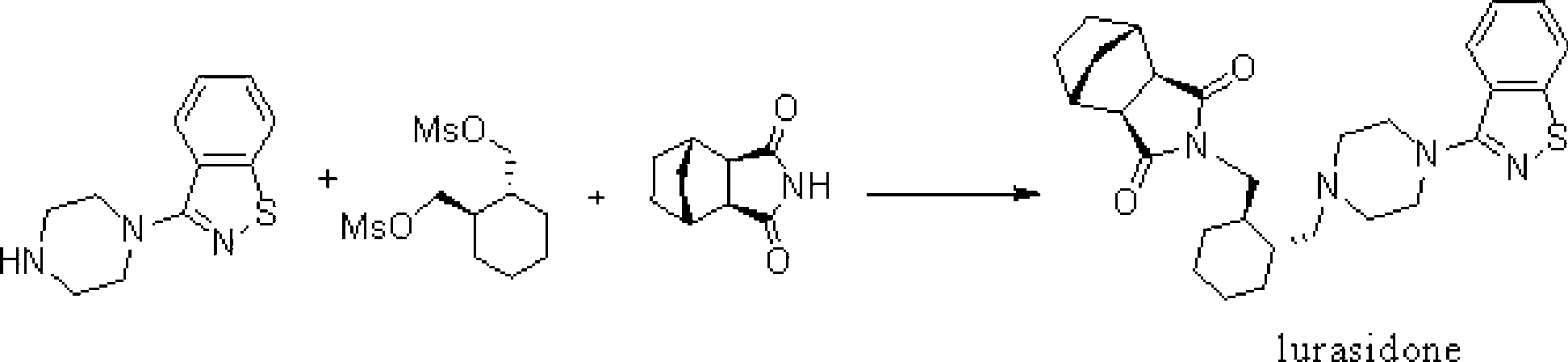
Synthetic method of lurasidone
A technology of lurasidone and a synthesis method, which is applied in the field of organic synthesis, can solve the problems of low synthesis efficiency, high cost, high consumption of raw materials, etc., and achieves the effects of reduced production cost, short reaction steps, and simple post-treatment process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] On a 1-liter four-neck flask, install a mechanical stirrer, a thermometer, and under nitrogen protection, add 62 grams of (R,R')-1,2-bis(methylsulfonyl-2oxymethyl)cyclohexane at room temperature (0.21mol), 44.6 grams of 3-(1-piperazinyl)-1,2-benzisothiazole (0.2mol), 500 milliliters of DMF, and 53 grams of Na 2 CO 3 (0.5mol). Then heat and stir, and react at 80° C. for 16 hours.
[0039] Then cool down to below 40°C, add 38.3g of bicyclo[2,2,1]heptane-2,3-dicarboximide (0.22mol) in batches to the reaction liquid, then raise the temperature to 80°C, and react 18 Hour. Cool to room temperature, filter, and distill off most of the DMF (about 450ml) from the filtrate at 70°C under reduced pressure. Cool to below 40°C, add 600ml of water to disperse the residual solid, filter at room temperature, and wash 3 times with 100ml of water to obtain a crude product. The crude product was dispersed in acetone for recrystallization, and dried to obtain 62 g of light yellow solid...
Embodiment 2
[0043] On the 3-liter four-neck flask, install a mechanical stirrer, a thermometer, and under nitrogen protection, add 187 grams of (R,R')-1,2-bis(methylsulfonyl-2oxymethyl)cyclohexane at room temperature (0.62mol, 1.02eq), 134 grams of 3-(1-piperazinyl)-1,2-benzisothiazole (0.6mol, 1.0eq), 2000 milliliters of ethylene glycol dimethyl ether, and 207 grams of K 2 CO 3 (1.5mol, 2.5eq). Then heat and stir, and react at 80° C. for 18 hours until the raw material bis(methylsulfonyl-2oxymethyl)cyclohexane disappears.
[0044] Then cool to below 40°C, add 109g of bicyclo[2,2,1]heptane-2,3-dicarboximide (0.66mol, 1.1eq.) in batches to the reaction liquid, and then heat up to 80°C , reacted for 16 hours. Cool and filter to remove inorganic salts and alkalis, distill most of the ethylene glycol dimethyl ether (about 1800ml) out of the filtrate at 60°C under reduced pressure, then cool to 40°C, add 1200ml of water to disperse the residual solid, filter at room temperature, wash with 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com