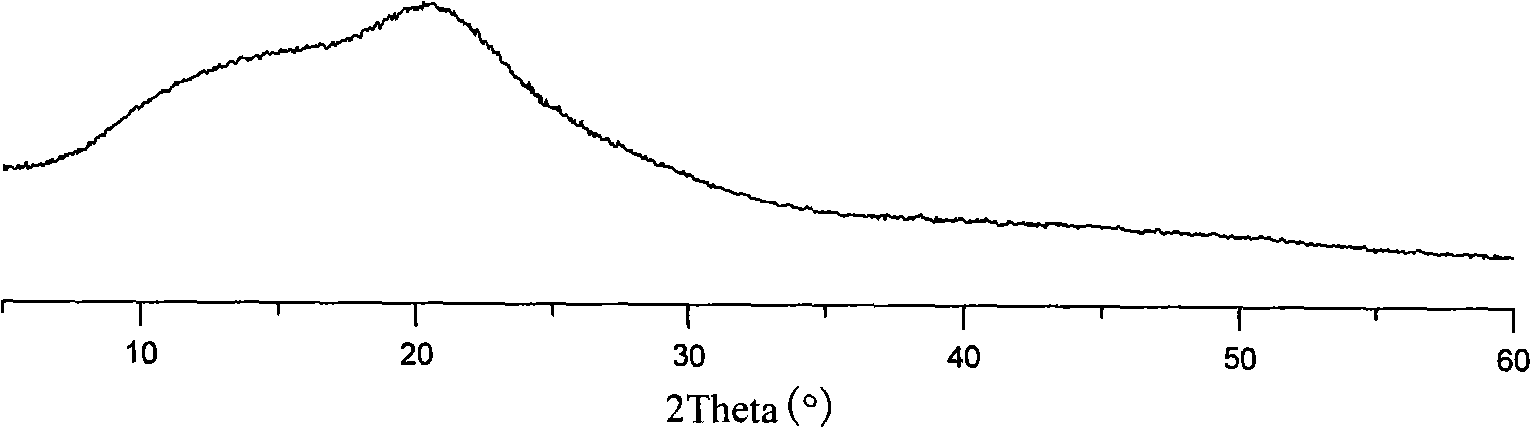
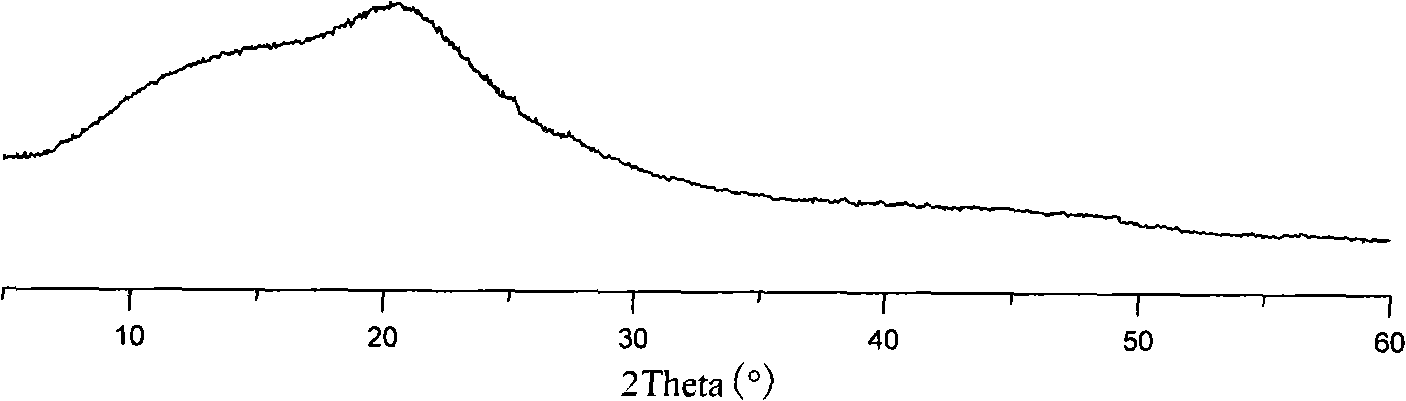
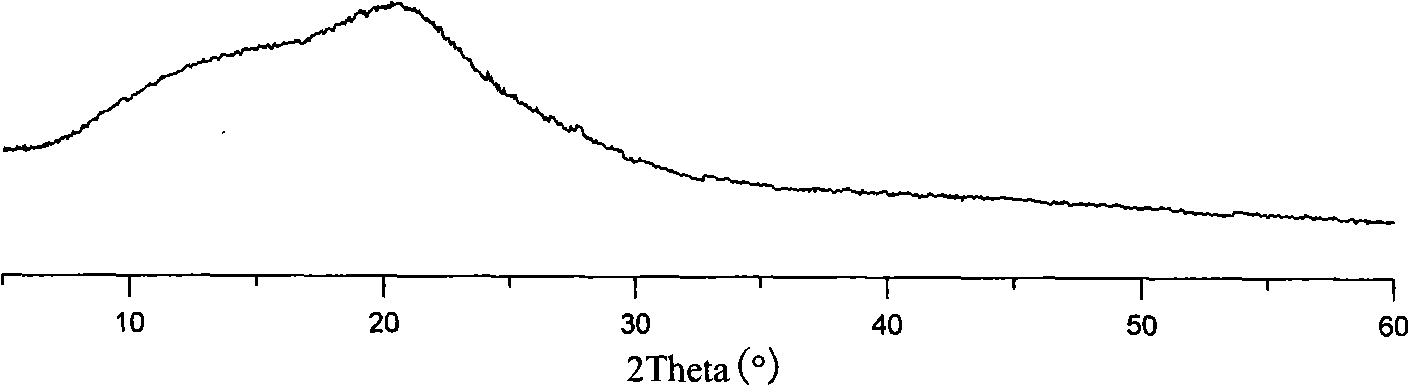
Pharmaceutical preparation containing benzimidazoles
A technology of benzimidazole and compound is applied in the field of pharmaceutical preparations containing benzimidazole compound solid dispersion, and can solve problems such as slow dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 enteric-coated pellet preparation containing lansoprazole
[0063] drug layer coating
[0064] The drug layer was added by weight as shown in Table 1 below.
[0065] Table 1 Drug layer formulation of lansoprazole enteric-coated pellets
[0066] Raw materials
Recipe 1
Recipe 2
Recipe 3
Recipe 4
Comparing formula 1 *
Comparing formula 2 *
blank core
250g
250g
250g
250g
250g
250g
100g
100g
100g
100g
100g
100g
PVPK30
150g
100g
80g
50g
200g
100g
50g
100g
70g
50g
0g
0g
15g
15g
15g
15g
15g
15g
[0067] * Note: the comparative formula is formulated according to the technical scheme disclosed in WO2006069159A2. According to the general knowledge of pharmacy, the ratio of medicine and hydrophilic carrier is less ...
Embodiment 2
[0107] Example 2 Enteric-coated pellet formulation containing esomeprazole
[0108] drug layer coating
[0109] Table 5 Drug layer formulation of esomeprazole enteric-coated pellets
[0110] Raw materials
Recipe 5
Recipe 6
Recipe 7
Recipe 8
blank core
250g
250g
250g
250g
80g
80g
80g
80g
PVP K30
160g
120g
100g
60g
0g
0g
20g
20g
10g
10g
10g
10g
[0111] The specific preparation method is as follows:
[0112] Take 0.9 liter of 50% ethanol, dissolve magnesium carbonate, add esomeprazole, stir until dissolved, add the prescribed amount of PVP K30 under stirring, and add twelve Sodium Alkyl Sulfate, stir until a homogeneous solution is formed.
[0113] Drug layer coating is carried out in a fluidized bed coating machine, and the specific operating parameters are according to Ex...
Embodiment 3
[0138] Example 3 Enteric-coated pellet preparation containing pantoprazole sodium
[0139] Drug Layer Formulation
[0140] Blank ball core 250g
[0141] Pantoprazole Sodium 110g
[0142] PVPK30 120g
[0143] Poloxamer 188 40g
[0144] Sodium carbonate 50g
[0145] The specific preparation method is as follows:
[0146] Take 1 liter of 50% ethanol, dissolve sodium carbonate, add pantoprazole sodium, stir until dissolved, add PVP K30 and poloxamer under stirring, and stir until forming a uniform solution. The blank pellet core is fluidized in a fluidized spray coating machine (DPL type micro-experimental machine), and the above-mentioned material liquid is spray-coated by adopting the bottom spray method. Concrete operating parameters are according to embodiment 1.
[0147] Barrier Coating
[0148] The pellets coated with the drug layer remain in a fluidized state and continue to be coated with an isolation layer.
[0149] The formula is as follows:
[0150] PVP-S630 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com