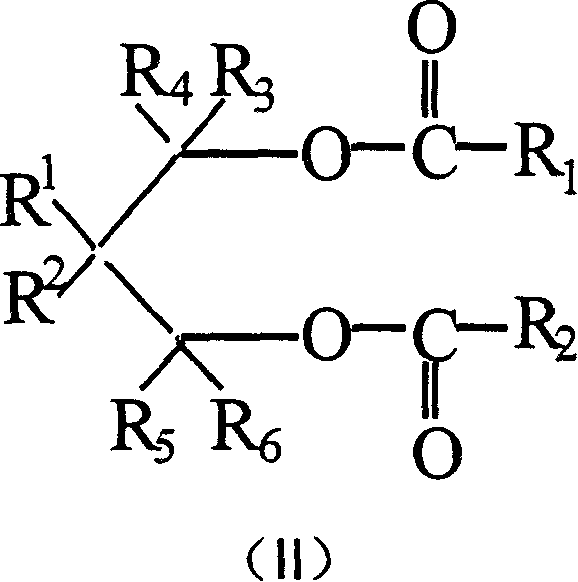
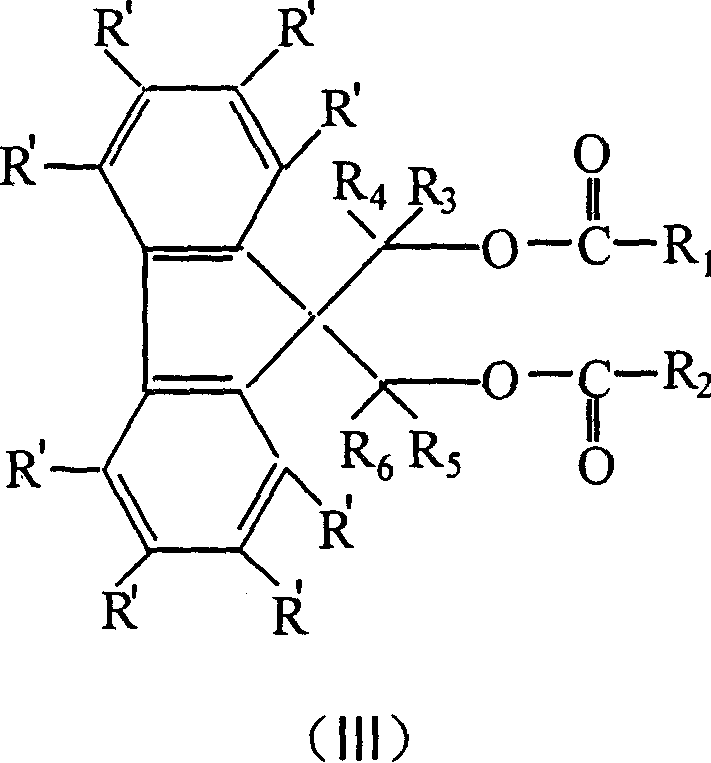
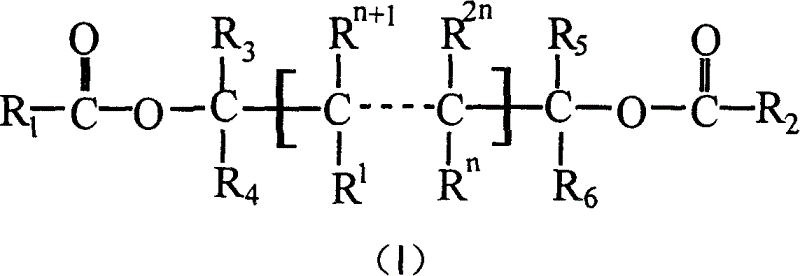Catalyst component for olefinic polymerization and its catalyst
A technology of olefin polymerization and catalyst, which is applied in the field of catalyst components and catalysts, and can solve the problems of unsatisfactory catalyst performance and unsatisfactory catalyst polymerization activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、9
[0050] Embodiment 1,9, the synthesis of 9-two (benzyl carboxymethyl) fluorene
[0051] (1) Synthesis of 9,9-dimethylolfluorene (see CN1141285A for the preparation method)
[0052] 9g (0.3mol) paraformaldehyde (vacuum dehydration 8h) was added 60ml dimethyl sulfoxide (DMSO, with CaH 2 Remove water) and the sodium ethylate solution after the reaction of 0.4gNa and 8ml absolute ethanol, and ice will be cooled. Under stirring, 100 ml of DMSO solution containing 16.6 g of fluorene (0.1 mol, dehydrated in vacuum for 8 h) was added within 30 seconds, and reacted for 10 min.
[0053] Add hydrochloric acid to terminate the reaction, and make the solution neutral, pour it into 300ml saturated brine, extract with ethyl acetate, separate the organic phase, wash twice with saturated brine, and dry over anhydrous sodium sulfate. The solvent was removed, and toluene was recrystallized to obtain 9,9-dimethylolfluorene as a white solid with a yield of 72%, m.p.142-145°C.
[0054] 1 H NMR (...
Embodiment 2
[0059] Embodiment 2, the synthesis of 2-isopropyl-2-isoamyl-1,3-propanediol benzhydryl carboxylate
[0060] (1) Synthesis of 2-isopropyl-5-methyl-2-hexenal (see CN1036846C for the preparation method).
[0061] 207g of isovaleraldehyde and 26ml of OH - Form Amberlite IRA910 resin (manufactured by Rohm & Hass) was heated to reflux. Remove the generated water with a water separator, stop the reaction after collecting about 26ml of water, and filter out the resin. Distill under reduced pressure and collect fractions at 85-90°C / 20mmHg.
[0062] (2) Synthesis of 2-isopropyl-5-methylhexanal
[0063] Add 70ml of ethanol, 1ml of saturated NaHCO to the 10g of 2-isopropyl-5-methyl-2-hexenal synthesized above 3 solution and 0.25 g of 10% Pd on carbon support. Access to N 2 , then pass into H 2 , means filled with graduated H 2 connected to the burette. The reaction was stirred at normal temperature and pressure until H 2 The absorption reaches the calculated value. Filter and u...
Embodiment 3、2
[0070] Embodiment 3,2, the preparation of 4-pentanediol dibenzoate
[0071] (1) Preparation of 2,4-pentanediol
[0072] A mixture of 10g of 2,4-pentanedione and 30ml of methanol was added dropwise to a mixed solution of 2.5g of sodium borohydride, 0.1g of sodium hydroxide and 25ml of water at 0-10°C. After the addition, the solvent was removed under reduced pressure and extracted continuously with 40ml of ethyl acetate for 15h. The solvent was removed and column chromatography was used to obtain 2,4-pentanediol as a colorless liquid with a yield of 90%.
[0073] (2) Preparation of 2,4-pentanediol dibenzoate
[0074] Add 30ml tetrahydrofuran and 0.09mol pyridine to 0.03mol 2,4-pentanediol, add 0.075mol benzoyl chloride under stirring, and heat to reflux for 4h. After cooling, add 20ml of saturated brine, extract with ethyl acetate, anhydrous Na 2 SO 4 Dry and remove solvent. Column chromatography or distillation under reduced pressure gave 2,4-pentanediol dibenzoate as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com