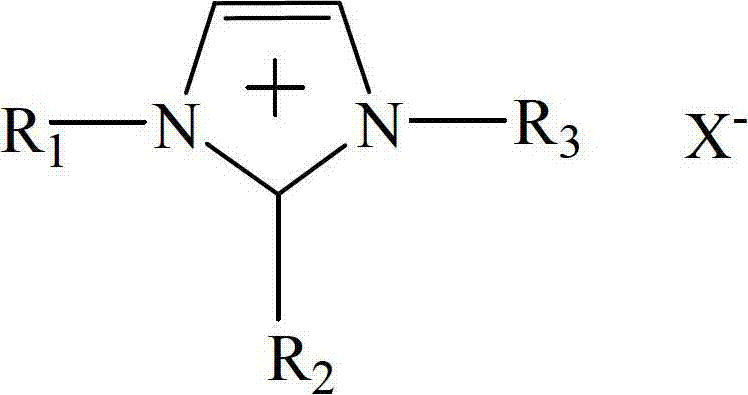
Method for synthesizing methylethyl carbonate by ester exchange of dimethyl carbonate and diethyl carbonate
A technology of diethyl carbonate and ethyl methyl carbonate, which is applied in the field of synthesizing ethyl methyl carbonate using ionic liquid as a catalyst, can solve the problems of high cost, poor selectivity, and low product yield, and achieve long life, High catalytic activity and cost reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add reaction material dimethyl carbonate and diethyl carbonate in the reactor, its mol ratio is 1:1, 1-butyl-2-methyl-3-ethylimidazolium bromide is as catalyzer, and used catalyst amount is The reactant is 1% of the mass of diethyl carbonate, the reaction temperature is 120°C, and the reaction time is 24h. Under these reaction conditions, the yield of ethyl methyl carbonate is 57.41%, and the selectivity is 100%.
Embodiment 2
[0026] Add reaction material dimethyl carbonate and diethyl carbonate in the reactor, its mol ratio is 1:1, and 1-methyl-2-butyl-3-methylimidazolium chloride is as catalyzer, and used catalyst amount is The mass of the reactant diethyl carbonate is 0.2%, the reaction temperature is 120°C, and the reaction time is 12h. Under these conditions, the yield of ethyl methyl carbonate is 37.58%, and the selectivity is 100%.
Embodiment 3
[0028] Add reaction material dimethyl carbonate and diethyl carbonate in the reactor, its molar ratio is 1:1, 1-butyl-2-methyl-3-butylimidazolium hexafluorophosphate is used as catalyst, and the amount of catalyst used It is 2% of the mass of the reactant diethyl carbonate, the reaction temperature is 100°C, and the reaction time is 20h. Under these reaction conditions, the yield of ethyl methyl carbonate is 22.15%, and the selectivity is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com