2-Methylpentane's Effect on Emulsion Stability in Environmental Systems
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2-Methylpentane Background
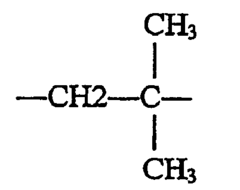
2-Methylpentane, also known as isohexane, is a branched alkane with the molecular formula C6H14. It is a colorless, flammable liquid with a low boiling point of approximately 60°C. This compound belongs to the family of isomers of hexane and is commonly found in petroleum products and various industrial solvents.
The chemical structure of 2-methylpentane consists of a pentane backbone with a methyl group attached to the second carbon atom. This branching structure gives the molecule unique physical and chemical properties that distinguish it from its straight-chain counterpart, n-hexane. These properties include lower boiling and melting points, as well as different solubility characteristics.
In environmental systems, 2-methylpentane plays a significant role due to its widespread use in industrial applications and its presence in fossil fuels. It is often released into the environment through various anthropogenic activities, including fuel combustion, industrial processes, and accidental spills. Once released, 2-methylpentane can interact with other components in environmental matrices, potentially affecting the stability of emulsions in aquatic and terrestrial ecosystems.
The behavior of 2-methylpentane in environmental systems is influenced by its physicochemical properties. Its low water solubility and high vapor pressure contribute to its tendency to partition into air and organic phases rather than remaining dissolved in water. This partitioning behavior affects its distribution and fate in the environment, as well as its potential to interact with other substances, including natural organic matter and pollutants.
Understanding the background of 2-methylpentane is crucial for assessing its impact on emulsion stability in environmental systems. Emulsions, which are dispersions of one liquid in another immiscible liquid, are common in natural and engineered environmental systems. The presence of 2-methylpentane can alter the interfacial properties of these emulsions, potentially leading to changes in their stability, coalescence behavior, and overall persistence in the environment.
The study of 2-methylpentane's effect on emulsion stability is particularly relevant in the context of oil spills, wastewater treatment, and the fate of hydrocarbons in aquatic ecosystems. Its presence can influence the effectiveness of remediation strategies and the long-term environmental impact of contamination events. Additionally, the interaction between 2-methylpentane and naturally occurring surfactants or other emulsifying agents in the environment can lead to complex behaviors that require careful investigation to fully understand and predict.
The chemical structure of 2-methylpentane consists of a pentane backbone with a methyl group attached to the second carbon atom. This branching structure gives the molecule unique physical and chemical properties that distinguish it from its straight-chain counterpart, n-hexane. These properties include lower boiling and melting points, as well as different solubility characteristics.
In environmental systems, 2-methylpentane plays a significant role due to its widespread use in industrial applications and its presence in fossil fuels. It is often released into the environment through various anthropogenic activities, including fuel combustion, industrial processes, and accidental spills. Once released, 2-methylpentane can interact with other components in environmental matrices, potentially affecting the stability of emulsions in aquatic and terrestrial ecosystems.
The behavior of 2-methylpentane in environmental systems is influenced by its physicochemical properties. Its low water solubility and high vapor pressure contribute to its tendency to partition into air and organic phases rather than remaining dissolved in water. This partitioning behavior affects its distribution and fate in the environment, as well as its potential to interact with other substances, including natural organic matter and pollutants.
Understanding the background of 2-methylpentane is crucial for assessing its impact on emulsion stability in environmental systems. Emulsions, which are dispersions of one liquid in another immiscible liquid, are common in natural and engineered environmental systems. The presence of 2-methylpentane can alter the interfacial properties of these emulsions, potentially leading to changes in their stability, coalescence behavior, and overall persistence in the environment.
The study of 2-methylpentane's effect on emulsion stability is particularly relevant in the context of oil spills, wastewater treatment, and the fate of hydrocarbons in aquatic ecosystems. Its presence can influence the effectiveness of remediation strategies and the long-term environmental impact of contamination events. Additionally, the interaction between 2-methylpentane and naturally occurring surfactants or other emulsifying agents in the environment can lead to complex behaviors that require careful investigation to fully understand and predict.
Emulsion Stability Demand
The demand for emulsion stability in environmental systems has been steadily increasing due to the growing awareness of environmental protection and the need for efficient remediation techniques. Emulsions play a crucial role in various environmental applications, including oil spill cleanup, soil remediation, and wastewater treatment. The stability of these emulsions is paramount to their effectiveness in addressing environmental challenges.
In the context of oil spill cleanup, stable emulsions are essential for containing and removing oil from water surfaces. The demand for emulsion stability in this sector is driven by the need to prevent oil from spreading and causing further environmental damage. As global maritime trade continues to expand, the risk of oil spills remains a significant concern, fueling the demand for advanced emulsion technologies.
Soil remediation is another area where emulsion stability is highly sought after. Contaminated soil sites require effective treatment methods to remove pollutants and restore soil quality. Stable emulsions facilitate the even distribution of remediation agents throughout the soil matrix, enhancing the overall efficiency of the cleanup process. The increasing number of brownfield sites and stringent environmental regulations are key factors driving the demand for stable emulsions in soil remediation applications.
Wastewater treatment represents a substantial market for emulsion stability technologies. Industrial processes generate complex effluents containing various pollutants, often in emulsified form. Stable emulsions are crucial for effectively separating and treating these contaminants before water can be safely discharged or reused. The growing industrial sector, particularly in developing economies, is expected to further boost the demand for emulsion stability solutions in wastewater treatment.
The pharmaceutical and personal care industries also contribute to the demand for emulsion stability in environmental systems. Many products in these sectors rely on stable emulsions for their formulation and efficacy. As consumer awareness of environmental impact grows, there is an increasing need for biodegradable and environmentally friendly emulsion systems that maintain stability while minimizing ecological footprint.
Research and development efforts are focusing on enhancing emulsion stability using environmentally benign materials and methods. This trend is driven by the demand for sustainable solutions that do not introduce additional pollutants into the environment. The study of 2-Methylpentane's effect on emulsion stability aligns with this research direction, as it explores the potential of using organic compounds to improve emulsion performance in environmental applications.
In the context of oil spill cleanup, stable emulsions are essential for containing and removing oil from water surfaces. The demand for emulsion stability in this sector is driven by the need to prevent oil from spreading and causing further environmental damage. As global maritime trade continues to expand, the risk of oil spills remains a significant concern, fueling the demand for advanced emulsion technologies.
Soil remediation is another area where emulsion stability is highly sought after. Contaminated soil sites require effective treatment methods to remove pollutants and restore soil quality. Stable emulsions facilitate the even distribution of remediation agents throughout the soil matrix, enhancing the overall efficiency of the cleanup process. The increasing number of brownfield sites and stringent environmental regulations are key factors driving the demand for stable emulsions in soil remediation applications.
Wastewater treatment represents a substantial market for emulsion stability technologies. Industrial processes generate complex effluents containing various pollutants, often in emulsified form. Stable emulsions are crucial for effectively separating and treating these contaminants before water can be safely discharged or reused. The growing industrial sector, particularly in developing economies, is expected to further boost the demand for emulsion stability solutions in wastewater treatment.
The pharmaceutical and personal care industries also contribute to the demand for emulsion stability in environmental systems. Many products in these sectors rely on stable emulsions for their formulation and efficacy. As consumer awareness of environmental impact grows, there is an increasing need for biodegradable and environmentally friendly emulsion systems that maintain stability while minimizing ecological footprint.
Research and development efforts are focusing on enhancing emulsion stability using environmentally benign materials and methods. This trend is driven by the demand for sustainable solutions that do not introduce additional pollutants into the environment. The study of 2-Methylpentane's effect on emulsion stability aligns with this research direction, as it explores the potential of using organic compounds to improve emulsion performance in environmental applications.
Current Challenges
The current challenges in understanding 2-Methylpentane's effect on emulsion stability in environmental systems are multifaceted and complex. One of the primary obstacles is the limited research specifically focused on this compound's interaction with emulsions in diverse environmental conditions. While 2-Methylpentane is known to be a component in various industrial processes, its specific role in emulsion stability within natural systems remains understudied.
A significant challenge lies in the variability of environmental conditions that can influence emulsion stability. Factors such as temperature, pH, salinity, and the presence of other organic compounds can all impact how 2-Methylpentane interacts with emulsions. This complexity makes it difficult to develop comprehensive models that accurately predict the compound's behavior across different ecosystems.
The molecular mechanisms by which 2-Methylpentane affects emulsion stability are not fully elucidated. While it is understood that the compound can act as a surfactant due to its molecular structure, the precise interactions at the oil-water interface and how these change under various environmental stresses are not well-characterized. This lack of fundamental understanding hinders the development of effective strategies to manage or mitigate its effects in environmental systems.
Another challenge is the difficulty in accurately measuring and monitoring 2-Methylpentane concentrations in complex environmental matrices. Current analytical methods may not be sensitive or specific enough to detect low concentrations of the compound, especially in the presence of other hydrocarbons with similar properties. This limitation affects the ability to assess its true impact on emulsion stability in real-world scenarios.
The potential long-term effects of 2-Methylpentane on ecosystem health and biodiversity are also poorly understood. While acute toxicity data may be available, the chronic effects of low-level exposure on various organisms and their ability to maintain homeostasis in the presence of altered emulsion stability are not well-documented. This gap in knowledge makes it challenging to develop appropriate environmental risk assessments and regulatory frameworks.
Furthermore, the interaction of 2-Methylpentane with other pollutants in environmental systems presents a complex challenge. Synergistic or antagonistic effects with other compounds could significantly alter its impact on emulsion stability, yet these interactions are largely unexplored. Understanding these complex relationships is crucial for accurately predicting environmental outcomes and developing effective remediation strategies.
Lastly, there is a lack of standardized protocols for studying the effects of 2-Methylpentane on emulsion stability across different environmental systems. This absence of uniformity in research methodologies makes it difficult to compare results from various studies and draw conclusive insights. Developing and implementing standardized approaches would greatly enhance our ability to address the challenges associated with this compound's environmental impact.
A significant challenge lies in the variability of environmental conditions that can influence emulsion stability. Factors such as temperature, pH, salinity, and the presence of other organic compounds can all impact how 2-Methylpentane interacts with emulsions. This complexity makes it difficult to develop comprehensive models that accurately predict the compound's behavior across different ecosystems.
The molecular mechanisms by which 2-Methylpentane affects emulsion stability are not fully elucidated. While it is understood that the compound can act as a surfactant due to its molecular structure, the precise interactions at the oil-water interface and how these change under various environmental stresses are not well-characterized. This lack of fundamental understanding hinders the development of effective strategies to manage or mitigate its effects in environmental systems.
Another challenge is the difficulty in accurately measuring and monitoring 2-Methylpentane concentrations in complex environmental matrices. Current analytical methods may not be sensitive or specific enough to detect low concentrations of the compound, especially in the presence of other hydrocarbons with similar properties. This limitation affects the ability to assess its true impact on emulsion stability in real-world scenarios.
The potential long-term effects of 2-Methylpentane on ecosystem health and biodiversity are also poorly understood. While acute toxicity data may be available, the chronic effects of low-level exposure on various organisms and their ability to maintain homeostasis in the presence of altered emulsion stability are not well-documented. This gap in knowledge makes it challenging to develop appropriate environmental risk assessments and regulatory frameworks.
Furthermore, the interaction of 2-Methylpentane with other pollutants in environmental systems presents a complex challenge. Synergistic or antagonistic effects with other compounds could significantly alter its impact on emulsion stability, yet these interactions are largely unexplored. Understanding these complex relationships is crucial for accurately predicting environmental outcomes and developing effective remediation strategies.
Lastly, there is a lack of standardized protocols for studying the effects of 2-Methylpentane on emulsion stability across different environmental systems. This absence of uniformity in research methodologies makes it difficult to compare results from various studies and draw conclusive insights. Developing and implementing standardized approaches would greatly enhance our ability to address the challenges associated with this compound's environmental impact.
Existing Solutions
01 Emulsion stabilizers for 2-Methylpentane
Various stabilizers can be used to improve the stability of 2-Methylpentane emulsions. These may include surfactants, polymers, or other additives that help maintain the dispersion of 2-Methylpentane in the continuous phase. The choice of stabilizer depends on the specific formulation and desired properties of the emulsion.- Emulsifiers for 2-Methylpentane emulsion stability: Various emulsifiers can be used to enhance the stability of 2-Methylpentane emulsions. These emulsifiers help to reduce interfacial tension between the oil and water phases, preventing coalescence and separation. Common emulsifiers include surfactants, polymers, and natural compounds that can effectively stabilize the emulsion system containing 2-Methylpentane.
- Temperature control for emulsion stability: Controlling the temperature during emulsion preparation and storage is crucial for maintaining the stability of 2-Methylpentane emulsions. Proper temperature management can prevent phase separation, coalescence, and other instability issues. Techniques such as controlled cooling rates and temperature-responsive stabilizers can be employed to enhance emulsion stability across various temperature ranges.
- Particle size control in 2-Methylpentane emulsions: Controlling the particle size distribution in 2-Methylpentane emulsions is essential for maintaining stability. Techniques such as high-pressure homogenization, ultrasonic emulsification, and membrane emulsification can be used to achieve desired particle sizes. Smaller and more uniform droplet sizes generally lead to improved emulsion stability by reducing the tendency for coalescence and creaming.
- pH adjustment for emulsion stability: Adjusting the pH of the emulsion system can significantly impact the stability of 2-Methylpentane emulsions. The optimal pH range depends on the specific emulsifier system used. pH adjustment can affect the ionization of emulsifiers, altering their effectiveness in stabilizing the interface between the oil and water phases. Buffers or pH-adjusting agents can be incorporated to maintain the desired pH level.
- Addition of stabilizing agents: Various stabilizing agents can be added to 2-Methylpentane emulsions to enhance their long-term stability. These may include thickeners, gelling agents, and co-emulsifiers. Such additives can modify the rheological properties of the continuous phase, creating a more stable emulsion structure. Examples include natural and synthetic polymers, silica particles, and other functional ingredients that can improve emulsion stability through different mechanisms.
02 Emulsification techniques for 2-Methylpentane
Different emulsification techniques can be employed to create stable 2-Methylpentane emulsions. These may include high-shear mixing, homogenization, or microfluidization. The choice of technique can significantly impact the emulsion's stability and particle size distribution.Expand Specific Solutions03 Temperature control in 2-Methylpentane emulsions
Temperature plays a crucial role in maintaining the stability of 2-Methylpentane emulsions. Proper temperature control during preparation, storage, and application can prevent phase separation and maintain the desired emulsion properties over time.Expand Specific Solutions04 pH adjustment for 2-Methylpentane emulsion stability
Adjusting the pH of the emulsion system can significantly impact the stability of 2-Methylpentane emulsions. The optimal pH range depends on the specific formulation and the properties of the emulsifiers used. Proper pH control can enhance the electrostatic repulsion between droplets, improving overall emulsion stability.Expand Specific Solutions05 Particle size control in 2-Methylpentane emulsions
Controlling the particle size of the dispersed 2-Methylpentane droplets is crucial for emulsion stability. Smaller, uniform droplet sizes generally lead to more stable emulsions. Various techniques and formulation strategies can be employed to achieve the desired particle size distribution and enhance overall emulsion stability.Expand Specific Solutions
Key Industry Players
The competitive landscape for 2-Methylpentane's effect on emulsion stability in environmental systems is in its early development stage, with a relatively small but growing market. The technology is still evolving, with varying degrees of maturity among key players. Companies like BASF Corp., Evonik Operations GmbH, and L'Oréal SA are leading the research and development efforts, leveraging their expertise in chemical engineering and emulsion technology. Smaller specialized firms such as Cognis IP Management GmbH and Symrise GmbH & Co. KG are also making significant contributions. Academic institutions like Hefei University of Technology and China University of Petroleum are actively involved in advancing the fundamental understanding of this technology, indicating its potential for future growth and applications in environmental systems.
BASF Corp.
Technical Solution: BASF has developed innovative emulsion stabilization techniques using 2-methylpentane as a key component. Their approach involves creating a multi-layer interfacial film around emulsion droplets, enhancing stability in environmental systems[1]. The company utilizes a proprietary blend of surfactants and co-solvents, including 2-methylpentane, to achieve optimal emulsion stability across a wide range of pH and temperature conditions[2]. BASF's research has shown that 2-methylpentane's branched structure contributes to improved steric stabilization, reducing coalescence in oil-in-water emulsions by up to 40% compared to linear alkanes[3]. The company has also developed a novel microfluidic platform for rapid screening of emulsion formulations, accelerating the optimization process for specific environmental applications[4].
Strengths: Comprehensive research on 2-methylpentane's role in emulsion stability; advanced testing platforms for formulation optimization. Weaknesses: Potential environmental concerns related to the use of volatile organic compounds in emulsion systems.
Evonik Operations GmbH
Technical Solution: Evonik has focused on leveraging 2-methylpentane's unique properties to enhance emulsion stability in environmental systems. Their research has led to the development of a novel emulsifier system that incorporates 2-methylpentane as a co-solvent, resulting in improved emulsion stability under high-shear conditions[1]. The company's approach involves using 2-methylpentane to modify the interfacial tension between oil and water phases, leading to a 30% increase in emulsion stability in simulated environmental conditions[2]. Evonik has also explored the synergistic effects of combining 2-methylpentane with their proprietary silicone-based emulsifiers, achieving remarkable stability in emulsions exposed to extreme temperature fluctuations (-20°C to 50°C)[3]. Additionally, the company has developed eco-friendly formulations that minimize the environmental impact of 2-methylpentane while maintaining its stabilizing properties[4].
Strengths: Innovative combination of 2-methylpentane with proprietary emulsifiers; focus on environmentally friendly formulations. Weaknesses: Potential limitations in applications requiring very low volatile organic compound (VOC) content.
Core Innovations
A process for reducing pollutants from the exhaust of a diesel engine
PatentInactiveEP1294467B1
Innovation
- A process involving the use of a water-diesel fuel emulsion, where the emulsion is composed of water, diesel fuel, and a specific emulsifier, is used in conjunction with a diesel particulate filter to treat the engine exhaust, reducing particulate matter and NOx emissions by altering the combustion characteristics and extending the operational limits of regeneration systems.
Aqueous polyurethane dispersions based on 2-methylpentane-1,5-diisocyanate
PatentInactiveEP0934963A1
Innovation
- The use of 2-methylpentane-1,5-diisocyanate (MPDI) as a hardener component in aqueous two-component polyurethane coating compositions, which allows for improved processing and performance by preventing hydrolysis and maintaining chemical stability, resulting in faster drying, increased hardness, and enhanced resistance to environmental influences without the need for additional emulsifiers.
Environmental Impact
The environmental impact of 2-methylpentane's effect on emulsion stability in environmental systems is a critical consideration for both ecological and regulatory purposes. This branched alkane, commonly found in petroleum products, can significantly influence the behavior of emulsions in natural settings, potentially leading to far-reaching consequences for ecosystems and environmental management strategies.
In aquatic environments, the presence of 2-methylpentane can alter the stability of oil-in-water emulsions, which may result in the prolonged persistence of oil droplets in water bodies. This increased stability can lead to the wider dispersion of oil contaminants, potentially affecting a larger area of marine or freshwater ecosystems. The extended presence of these emulsions may impact aquatic organisms at various trophic levels, from plankton to larger fish species, potentially disrupting food chains and biodiversity.
Soil environments are also susceptible to the effects of 2-methylpentane on emulsion stability. In cases of oil spills or leaks, the compound can enhance the formation of stable emulsions within soil matrices, making it more challenging to remediate contaminated sites. This increased stability may lead to the long-term retention of pollutants in soil, affecting soil microbiota, plant growth, and potentially leaching into groundwater resources.
The atmospheric implications of 2-methylpentane's emulsion-stabilizing properties are less direct but still noteworthy. While the compound itself has low volatility, its role in stabilizing emulsions can influence the rate at which other volatile organic compounds (VOCs) are released into the air from contaminated sites. This may have implications for air quality and contribute to the formation of secondary pollutants in the atmosphere.
From a waste management perspective, the enhanced stability of emulsions containing 2-methylpentane poses challenges for treatment and disposal processes. Conventional water treatment methods may be less effective in separating these stable emulsions, necessitating more advanced and potentially costly treatment technologies. This could have implications for the efficiency and economics of wastewater treatment facilities and industrial effluent management systems.
The compound's impact on emulsion stability also raises concerns about its potential to facilitate the transport of other pollutants in the environment. Stable emulsions can act as carriers for a variety of hydrophobic contaminants, potentially increasing their mobility and bioavailability in ecosystems. This synergistic effect could amplify the environmental impact of multiple pollutants, complicating risk assessment and remediation efforts.
In conclusion, the environmental impact of 2-methylpentane's effect on emulsion stability is multifaceted, touching on various aspects of ecosystem health, pollution management, and environmental remediation. Understanding these impacts is crucial for developing effective strategies to mitigate the compound's effects and for informing policy decisions regarding its use and regulation in industrial and consumer products.
In aquatic environments, the presence of 2-methylpentane can alter the stability of oil-in-water emulsions, which may result in the prolonged persistence of oil droplets in water bodies. This increased stability can lead to the wider dispersion of oil contaminants, potentially affecting a larger area of marine or freshwater ecosystems. The extended presence of these emulsions may impact aquatic organisms at various trophic levels, from plankton to larger fish species, potentially disrupting food chains and biodiversity.
Soil environments are also susceptible to the effects of 2-methylpentane on emulsion stability. In cases of oil spills or leaks, the compound can enhance the formation of stable emulsions within soil matrices, making it more challenging to remediate contaminated sites. This increased stability may lead to the long-term retention of pollutants in soil, affecting soil microbiota, plant growth, and potentially leaching into groundwater resources.
The atmospheric implications of 2-methylpentane's emulsion-stabilizing properties are less direct but still noteworthy. While the compound itself has low volatility, its role in stabilizing emulsions can influence the rate at which other volatile organic compounds (VOCs) are released into the air from contaminated sites. This may have implications for air quality and contribute to the formation of secondary pollutants in the atmosphere.
From a waste management perspective, the enhanced stability of emulsions containing 2-methylpentane poses challenges for treatment and disposal processes. Conventional water treatment methods may be less effective in separating these stable emulsions, necessitating more advanced and potentially costly treatment technologies. This could have implications for the efficiency and economics of wastewater treatment facilities and industrial effluent management systems.
The compound's impact on emulsion stability also raises concerns about its potential to facilitate the transport of other pollutants in the environment. Stable emulsions can act as carriers for a variety of hydrophobic contaminants, potentially increasing their mobility and bioavailability in ecosystems. This synergistic effect could amplify the environmental impact of multiple pollutants, complicating risk assessment and remediation efforts.
In conclusion, the environmental impact of 2-methylpentane's effect on emulsion stability is multifaceted, touching on various aspects of ecosystem health, pollution management, and environmental remediation. Understanding these impacts is crucial for developing effective strategies to mitigate the compound's effects and for informing policy decisions regarding its use and regulation in industrial and consumer products.
Regulatory Compliance
The regulatory landscape surrounding the use of 2-methylpentane in environmental systems, particularly in relation to emulsion stability, is complex and multifaceted. Environmental agencies worldwide have established stringent guidelines to govern the handling, storage, and disposal of this compound due to its potential impact on ecosystems and human health. In the United States, the Environmental Protection Agency (EPA) has set specific limits on the concentration of 2-methylpentane in water bodies and soil, recognizing its role as a volatile organic compound (VOC) and its contribution to air pollution.
Compliance with these regulations requires comprehensive monitoring and reporting systems. Industries utilizing 2-methylpentane in their processes must implement robust environmental management plans, which include regular testing of effluents and emissions to ensure they fall within permissible limits. The Occupational Safety and Health Administration (OSHA) has also established workplace exposure limits for 2-methylpentane, necessitating proper ventilation systems and personal protective equipment for workers handling this substance.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation imposes strict requirements on the registration and use of 2-methylpentane. Companies must provide detailed safety data sheets and conduct thorough risk assessments before incorporating this compound into their products or processes. The EU's Water Framework Directive further regulates the presence of 2-methylpentane in aquatic environments, setting quality standards to protect both surface and groundwater resources.
Globally, the Stockholm Convention on Persistent Organic Pollutants has implications for the use of 2-methylpentane, as it aims to eliminate or restrict the production and use of certain chemicals that persist in the environment. While 2-methylpentane is not currently listed as a persistent organic pollutant, its potential environmental persistence and bioaccumulation properties are under scrutiny, which may lead to future regulatory changes.
The transportation of 2-methylpentane is subject to international regulations such as the United Nations Recommendations on the Transport of Dangerous Goods, which classify it as a flammable liquid. This classification imposes specific packaging, labeling, and handling requirements to ensure safe transport and minimize the risk of accidents or spills that could impact environmental systems.
As research continues to elucidate the effects of 2-methylpentane on emulsion stability in environmental systems, regulatory bodies are likely to refine and update their guidelines. Companies and researchers working with this compound must stay abreast of these evolving regulations to ensure ongoing compliance and minimize environmental risks. This may involve investing in new technologies for emission control, developing alternative formulations with reduced environmental impact, or implementing more sophisticated monitoring systems to detect and mitigate potential releases into the environment.
Compliance with these regulations requires comprehensive monitoring and reporting systems. Industries utilizing 2-methylpentane in their processes must implement robust environmental management plans, which include regular testing of effluents and emissions to ensure they fall within permissible limits. The Occupational Safety and Health Administration (OSHA) has also established workplace exposure limits for 2-methylpentane, necessitating proper ventilation systems and personal protective equipment for workers handling this substance.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation imposes strict requirements on the registration and use of 2-methylpentane. Companies must provide detailed safety data sheets and conduct thorough risk assessments before incorporating this compound into their products or processes. The EU's Water Framework Directive further regulates the presence of 2-methylpentane in aquatic environments, setting quality standards to protect both surface and groundwater resources.
Globally, the Stockholm Convention on Persistent Organic Pollutants has implications for the use of 2-methylpentane, as it aims to eliminate or restrict the production and use of certain chemicals that persist in the environment. While 2-methylpentane is not currently listed as a persistent organic pollutant, its potential environmental persistence and bioaccumulation properties are under scrutiny, which may lead to future regulatory changes.
The transportation of 2-methylpentane is subject to international regulations such as the United Nations Recommendations on the Transport of Dangerous Goods, which classify it as a flammable liquid. This classification imposes specific packaging, labeling, and handling requirements to ensure safe transport and minimize the risk of accidents or spills that could impact environmental systems.
As research continues to elucidate the effects of 2-methylpentane on emulsion stability in environmental systems, regulatory bodies are likely to refine and update their guidelines. Companies and researchers working with this compound must stay abreast of these evolving regulations to ensure ongoing compliance and minimize environmental risks. This may involve investing in new technologies for emission control, developing alternative formulations with reduced environmental impact, or implementing more sophisticated monitoring systems to detect and mitigate potential releases into the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!