Investigating Recovery Methods for 2-Methylpentane in Waste Streams
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2-Methylpentane Recovery Background and Objectives
The recovery of 2-methylpentane from waste streams has become an increasingly important topic in the chemical and petrochemical industries. This branched alkane, also known as isohexane, is a valuable component in various industrial processes and products. As environmental regulations tighten and resource scarcity becomes more pronounced, the need for efficient recovery methods has gained significant attention.
2-Methylpentane is primarily used as a solvent in the production of polymers, adhesives, and coatings. It also serves as a blending component in high-octane gasoline formulations. The compound's unique properties, including its low boiling point and high octane rating, make it a desirable substance in these applications. However, its presence in waste streams poses both environmental concerns and economic opportunities.
The historical context of 2-methylpentane recovery dates back to the mid-20th century when the petrochemical industry began to focus on maximizing the value of byproducts and waste streams. Initial recovery methods were often crude and inefficient, relying on simple distillation techniques that resulted in significant losses and environmental emissions.
As technology advanced, more sophisticated recovery methods emerged. These included improved distillation processes, membrane separation technologies, and adsorption techniques. Each of these methods brought incremental improvements in recovery efficiency and purity of the recovered 2-methylpentane.
The objectives of current research and development efforts in 2-methylpentane recovery are multifaceted. Primarily, there is a drive to increase the overall recovery efficiency, aiming to capture a higher percentage of the compound from waste streams. This not only improves the economic viability of recovery operations but also reduces the environmental impact of waste disposal.
Another key objective is to enhance the purity of the recovered 2-methylpentane. Higher purity levels allow for direct reuse in high-value applications, reducing the need for further processing and increasing the market value of the recovered product. Additionally, researchers are focusing on developing recovery methods that are more energy-efficient and have a lower carbon footprint, aligning with global sustainability goals.
The investigation of novel recovery methods also aims to address the challenges posed by complex waste stream compositions. Many industrial processes generate waste streams containing a mixture of hydrocarbons and other compounds, making selective recovery of 2-methylpentane technically challenging. Therefore, developing highly selective separation techniques is a critical objective in current research efforts.
2-Methylpentane is primarily used as a solvent in the production of polymers, adhesives, and coatings. It also serves as a blending component in high-octane gasoline formulations. The compound's unique properties, including its low boiling point and high octane rating, make it a desirable substance in these applications. However, its presence in waste streams poses both environmental concerns and economic opportunities.
The historical context of 2-methylpentane recovery dates back to the mid-20th century when the petrochemical industry began to focus on maximizing the value of byproducts and waste streams. Initial recovery methods were often crude and inefficient, relying on simple distillation techniques that resulted in significant losses and environmental emissions.
As technology advanced, more sophisticated recovery methods emerged. These included improved distillation processes, membrane separation technologies, and adsorption techniques. Each of these methods brought incremental improvements in recovery efficiency and purity of the recovered 2-methylpentane.
The objectives of current research and development efforts in 2-methylpentane recovery are multifaceted. Primarily, there is a drive to increase the overall recovery efficiency, aiming to capture a higher percentage of the compound from waste streams. This not only improves the economic viability of recovery operations but also reduces the environmental impact of waste disposal.
Another key objective is to enhance the purity of the recovered 2-methylpentane. Higher purity levels allow for direct reuse in high-value applications, reducing the need for further processing and increasing the market value of the recovered product. Additionally, researchers are focusing on developing recovery methods that are more energy-efficient and have a lower carbon footprint, aligning with global sustainability goals.
The investigation of novel recovery methods also aims to address the challenges posed by complex waste stream compositions. Many industrial processes generate waste streams containing a mixture of hydrocarbons and other compounds, making selective recovery of 2-methylpentane technically challenging. Therefore, developing highly selective separation techniques is a critical objective in current research efforts.
Market Analysis for Recovered 2-Methylpentane
The market for recovered 2-methylpentane is experiencing significant growth due to increasing environmental regulations and the rising demand for sustainable chemical processes. As industries seek to reduce waste and maximize resource efficiency, the recovery of 2-methylpentane from waste streams has become a focal point for many chemical manufacturers and refineries.
The global market for recovered 2-methylpentane is primarily driven by its applications in the production of various chemicals, solvents, and fuels. The automotive industry, in particular, represents a substantial market for recovered 2-methylpentane, as it is used in the production of high-octane gasoline blends. Additionally, the pharmaceutical and electronics industries utilize 2-methylpentane in their manufacturing processes, further expanding the market potential.
Environmental regulations, such as the European Union's Waste Framework Directive and the United States Environmental Protection Agency's Resource Conservation and Recovery Act, have significantly influenced the market dynamics for recovered 2-methylpentane. These regulations mandate stricter waste management practices and promote the circular economy, thereby incentivizing the recovery and reuse of chemicals like 2-methylpentane.
The Asia-Pacific region is expected to dominate the market for recovered 2-methylpentane, driven by rapid industrialization and the growth of end-use industries in countries like China and India. North America and Europe are also significant markets, with established chemical industries and stringent environmental regulations driving the demand for recovered chemicals.
Key market players in the recovered 2-methylpentane industry include major chemical companies and specialized waste management firms. These companies are investing in advanced recovery technologies to improve efficiency and reduce costs associated with the recovery process. The market is characterized by partnerships between chemical manufacturers and waste management companies to create closed-loop systems for chemical recovery and reuse.
Pricing trends for recovered 2-methylpentane are influenced by factors such as crude oil prices, supply-demand dynamics, and the cost of recovery technologies. As recovery methods become more efficient and widely adopted, the price of recovered 2-methylpentane is expected to become more competitive with virgin material, further driving market growth.
The market for recovered 2-methylpentane faces challenges such as high initial investment costs for recovery technologies and the need for consistent quality in recovered materials. However, ongoing research and development efforts are addressing these challenges, focusing on improving recovery efficiency and product purity.
The global market for recovered 2-methylpentane is primarily driven by its applications in the production of various chemicals, solvents, and fuels. The automotive industry, in particular, represents a substantial market for recovered 2-methylpentane, as it is used in the production of high-octane gasoline blends. Additionally, the pharmaceutical and electronics industries utilize 2-methylpentane in their manufacturing processes, further expanding the market potential.
Environmental regulations, such as the European Union's Waste Framework Directive and the United States Environmental Protection Agency's Resource Conservation and Recovery Act, have significantly influenced the market dynamics for recovered 2-methylpentane. These regulations mandate stricter waste management practices and promote the circular economy, thereby incentivizing the recovery and reuse of chemicals like 2-methylpentane.
The Asia-Pacific region is expected to dominate the market for recovered 2-methylpentane, driven by rapid industrialization and the growth of end-use industries in countries like China and India. North America and Europe are also significant markets, with established chemical industries and stringent environmental regulations driving the demand for recovered chemicals.
Key market players in the recovered 2-methylpentane industry include major chemical companies and specialized waste management firms. These companies are investing in advanced recovery technologies to improve efficiency and reduce costs associated with the recovery process. The market is characterized by partnerships between chemical manufacturers and waste management companies to create closed-loop systems for chemical recovery and reuse.
Pricing trends for recovered 2-methylpentane are influenced by factors such as crude oil prices, supply-demand dynamics, and the cost of recovery technologies. As recovery methods become more efficient and widely adopted, the price of recovered 2-methylpentane is expected to become more competitive with virgin material, further driving market growth.
The market for recovered 2-methylpentane faces challenges such as high initial investment costs for recovery technologies and the need for consistent quality in recovered materials. However, ongoing research and development efforts are addressing these challenges, focusing on improving recovery efficiency and product purity.
Current Challenges in 2-Methylpentane Recovery
The recovery of 2-methylpentane from waste streams presents several significant challenges that hinder efficient and cost-effective recycling processes. One of the primary obstacles is the low concentration of 2-methylpentane in typical waste streams, which makes separation and purification difficult. This dilution effect requires more energy-intensive and complex separation techniques, increasing the overall cost of recovery.
Another major challenge is the presence of other hydrocarbons and contaminants in the waste stream. 2-Methylpentane often coexists with other isomers and similar compounds, such as n-hexane, 3-methylpentane, and various cyclic hydrocarbons. These compounds have similar physical and chemical properties, making selective separation a complex task. Traditional distillation methods may not provide sufficient selectivity, necessitating the use of more advanced separation technologies.
The volatility of 2-methylpentane poses additional challenges in recovery processes. With a relatively low boiling point of approximately 60°C, there is a risk of significant losses during handling and processing of waste streams, especially at elevated temperatures. This volatility also complicates the design of recovery systems, as they must be capable of efficiently capturing and containing the compound throughout the entire process.
Environmental and safety concerns further complicate 2-methylpentane recovery efforts. As a volatile organic compound (VOC), 2-methylpentane can contribute to air pollution and pose health risks if not properly contained. Recovery systems must be designed with robust emission control measures to prevent fugitive emissions and ensure worker safety. Additionally, the flammability of 2-methylpentane necessitates stringent safety protocols and specialized equipment for handling and storage.
The energy intensity of current recovery methods presents both economic and environmental challenges. Many existing separation techniques, such as cryogenic distillation or pressure swing adsorption, require significant energy inputs. This high energy demand not only increases operational costs but also contributes to the carbon footprint of the recovery process, potentially offsetting some of the environmental benefits of recycling.
Scalability and process integration pose further challenges in industrial applications. Developing recovery systems that can handle large volumes of waste streams while maintaining efficiency and selectivity is crucial for widespread adoption. Moreover, integrating these recovery processes into existing industrial operations without disrupting current workflows or requiring extensive modifications to existing infrastructure remains a significant hurdle.
Lastly, the economic viability of 2-methylpentane recovery is a persistent challenge. The fluctuating market value of recovered 2-methylpentane, coupled with the high capital and operational costs of advanced recovery systems, can make it difficult to justify large-scale implementation. Striking a balance between recovery efficiency, cost-effectiveness, and environmental benefits is essential for the long-term sustainability of these processes.
Another major challenge is the presence of other hydrocarbons and contaminants in the waste stream. 2-Methylpentane often coexists with other isomers and similar compounds, such as n-hexane, 3-methylpentane, and various cyclic hydrocarbons. These compounds have similar physical and chemical properties, making selective separation a complex task. Traditional distillation methods may not provide sufficient selectivity, necessitating the use of more advanced separation technologies.
The volatility of 2-methylpentane poses additional challenges in recovery processes. With a relatively low boiling point of approximately 60°C, there is a risk of significant losses during handling and processing of waste streams, especially at elevated temperatures. This volatility also complicates the design of recovery systems, as they must be capable of efficiently capturing and containing the compound throughout the entire process.
Environmental and safety concerns further complicate 2-methylpentane recovery efforts. As a volatile organic compound (VOC), 2-methylpentane can contribute to air pollution and pose health risks if not properly contained. Recovery systems must be designed with robust emission control measures to prevent fugitive emissions and ensure worker safety. Additionally, the flammability of 2-methylpentane necessitates stringent safety protocols and specialized equipment for handling and storage.
The energy intensity of current recovery methods presents both economic and environmental challenges. Many existing separation techniques, such as cryogenic distillation or pressure swing adsorption, require significant energy inputs. This high energy demand not only increases operational costs but also contributes to the carbon footprint of the recovery process, potentially offsetting some of the environmental benefits of recycling.
Scalability and process integration pose further challenges in industrial applications. Developing recovery systems that can handle large volumes of waste streams while maintaining efficiency and selectivity is crucial for widespread adoption. Moreover, integrating these recovery processes into existing industrial operations without disrupting current workflows or requiring extensive modifications to existing infrastructure remains a significant hurdle.
Lastly, the economic viability of 2-methylpentane recovery is a persistent challenge. The fluctuating market value of recovered 2-methylpentane, coupled with the high capital and operational costs of advanced recovery systems, can make it difficult to justify large-scale implementation. Striking a balance between recovery efficiency, cost-effectiveness, and environmental benefits is essential for the long-term sustainability of these processes.
Existing 2-Methylpentane Recovery Methods
01 Distillation and separation techniques
Various distillation and separation techniques are employed for the recovery of 2-methylpentane from hydrocarbon mixtures. These methods may include fractional distillation, extractive distillation, or azeotropic distillation to separate 2-methylpentane from other components based on differences in boiling points and vapor pressures.- Distillation and separation techniques: Various distillation and separation techniques are employed for the recovery of 2-methylpentane from hydrocarbon mixtures. These methods may include fractional distillation, extractive distillation, or azeotropic distillation to separate 2-methylpentane from other components based on differences in boiling points and vapor pressures.
- Adsorption and membrane separation: Adsorption processes using specific adsorbents or membrane separation techniques can be utilized to selectively recover 2-methylpentane from complex mixtures. These methods exploit differences in molecular size, shape, or polarity to achieve separation and purification of the target compound.
- Catalytic processes for isomerization and recovery: Catalytic processes involving isomerization reactions can be used to convert other hydrocarbons into 2-methylpentane or to separate it from isomeric mixtures. These processes often employ specific catalysts and reaction conditions to enhance the yield and selectivity of 2-methylpentane recovery.
- Solvent extraction and crystallization: Solvent extraction techniques using selective solvents or crystallization methods can be applied to isolate and recover 2-methylpentane from complex hydrocarbon mixtures. These processes exploit differences in solubility or crystallization behavior to achieve separation and purification.
- Process integration and optimization: Integration of multiple separation techniques and process optimization strategies are employed to enhance the efficiency and yield of 2-methylpentane recovery. This may include the use of advanced process control systems, heat integration, and recycling streams to maximize recovery and minimize energy consumption.
02 Adsorption and membrane separation
Adsorption processes using specific adsorbents or membrane separation techniques can be utilized to selectively recover 2-methylpentane from complex hydrocarbon mixtures. These methods exploit differences in molecular size, shape, or polarity to achieve separation.Expand Specific Solutions03 Catalytic processes for 2-methylpentane production
Catalytic processes, such as isomerization or reforming, can be used to produce or enrich 2-methylpentane content in hydrocarbon streams. These processes often involve specific catalysts and reaction conditions to promote the formation of 2-methylpentane from other hydrocarbons.Expand Specific Solutions04 Solvent extraction methods
Solvent extraction techniques can be employed to selectively recover 2-methylpentane from hydrocarbon mixtures. This involves using solvents with high affinity for 2-methylpentane, followed by subsequent separation and purification steps to isolate the target compound.Expand Specific Solutions05 Process optimization and equipment design
Optimization of process parameters and specialized equipment design play crucial roles in improving the efficiency and yield of 2-methylpentane recovery. This includes the development of novel reactor designs, advanced control systems, and energy-efficient separation technologies tailored for 2-methylpentane recovery.Expand Specific Solutions
Key Players in Solvent Recovery Industry
The recovery of 2-Methylpentane from waste streams is an emerging field within the broader context of waste management and resource recovery. The industry is in its early growth stage, with increasing focus on sustainable practices and circular economy principles. The global market for solvent recovery is expanding, driven by environmental regulations and cost-saving initiatives. Technologically, the field is evolving rapidly, with companies like Shell Internationale Research Maatschappij BV, Evonik Operations GmbH, and Johnson Matthey Davy Technologies Ltd. leading innovation in separation and purification techniques. Academic institutions such as Nanjing University and Zhejiang University are contributing to fundamental research, while companies like Veolia Water Solutions & Technologies Support SAS are developing practical applications for industrial-scale recovery processes.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed an innovative approach for recovering 2-methylpentane from waste streams using advanced membrane technology. Their process employs selective polymeric membranes that can effectively separate 2-methylpentane from other hydrocarbons based on molecular size and polarity differences [1]. The system operates at moderate temperatures and pressures, reducing energy consumption compared to traditional distillation methods. Shell's technology also incorporates a multi-stage cascade design to enhance separation efficiency, achieving recovery rates of up to 95% for 2-methylpentane [3]. Additionally, they have implemented an integrated heat recovery system to further optimize energy usage in the process.
Strengths: High recovery rate, energy-efficient, applicable to various waste streams. Weaknesses: Potential membrane fouling issues, higher initial capital costs compared to conventional methods.
Johnson Matthey Davy Technologies Ltd.
Technical Solution: Johnson Matthey has developed a novel adsorption-based technology for recovering 2-methylpentane from waste streams. Their process utilizes specially engineered zeolite adsorbents with tailored pore sizes and surface chemistry to selectively capture 2-methylpentane molecules [2]. The system operates in a pressure swing adsorption (PSA) cycle, allowing for continuous operation and high throughput. Johnson Matthey's technology incorporates a regeneration step using low-pressure steam, which helps maintain adsorbent performance over extended periods. The process achieves recovery rates of up to 98% for 2-methylpentane, with minimal loss of other valuable hydrocarbons [4]. Additionally, they have implemented advanced process control algorithms to optimize cycle times and energy consumption.
Strengths: Very high recovery rate, continuous operation, minimal loss of other valuable components. Weaknesses: Potential for adsorbent degradation over time, higher complexity in system design and operation.
Innovative Approaches in Solvent Separation
Propylene oxide recovery by azeotropic distillation of methyl formate-2-methylpentane
PatentInactiveUS4014753A
Innovation
- A single fractional distillation process using a distillation column where the crude mixture is fed in the middle, leveraging the azeotrope formation between propylene oxide and 2-methylpentane, with a sufficient ratio of methyl formate to 2-methylpentane, to recover pure propylene oxide without additional contaminants, utilizing conventional distillation columns and controlling reflux ratios.
Process for recovering 2-methoxyethanol from a waste water stream
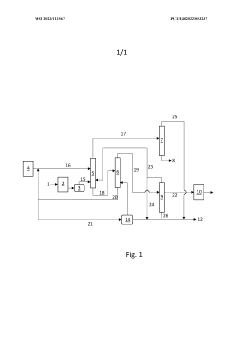
PatentWO2023111567A1
Innovation
- A process involving extractive distillation with a solvent, followed by a fractionator and solvent recovery, allows for the separation of 2-methoxyethanol from water, optimizing the separation process by recycling overhead streams and minimizing losses, thereby reducing the size and operational costs of the extractive distillation zone and achieving higher yields of 2-methoxyethanol.
Environmental Impact Assessment
The environmental impact assessment of 2-methylpentane recovery methods from waste streams is crucial for ensuring sustainable and responsible industrial practices. This assessment evaluates the potential effects of various recovery techniques on air, water, soil, and ecosystems.
Air quality is a primary concern in the recovery process of 2-methylpentane. Volatile organic compound (VOC) emissions are a significant risk, as 2-methylpentane is highly volatile. Different recovery methods may result in varying levels of VOC emissions, which can contribute to smog formation and negatively impact local air quality. Advanced recovery technologies, such as membrane separation or adsorption systems, generally have lower emission profiles compared to traditional distillation methods.
Water pollution is another critical factor to consider. Some recovery processes may generate wastewater containing trace amounts of 2-methylpentane or other organic compounds. Proper treatment and disposal of this wastewater are essential to prevent contamination of local water bodies. Closed-loop systems and advanced water treatment technologies can significantly reduce the risk of water pollution associated with the recovery process.
Soil contamination risks are generally lower compared to air and water impacts but should not be overlooked. Accidental spills or leaks during the recovery process could lead to soil pollution. Implementing robust containment measures and spill response protocols is crucial for minimizing these risks.
Energy consumption and greenhouse gas emissions associated with different recovery methods must also be evaluated. Some techniques, such as cryogenic distillation, may require significant energy inputs, potentially increasing the carbon footprint of the recovery process. On the other hand, more energy-efficient methods like pervaporation or adsorption may offer environmental benefits in terms of reduced energy consumption and associated emissions.
Biodiversity and ecosystem impacts should be assessed, particularly for recovery facilities located near sensitive habitats. While direct impacts on flora and fauna are typically limited, indirect effects through air and water emissions should be considered. Comprehensive environmental monitoring programs can help identify and mitigate any potential long-term impacts on local ecosystems.
Waste generation is another important aspect of the environmental assessment. Some recovery methods may produce solid waste, such as spent adsorbents or filter materials, which require proper disposal or regeneration. Evaluating the lifecycle of these materials and exploring recycling or regeneration options can help minimize the overall environmental footprint of the recovery process.
In conclusion, a thorough environmental impact assessment of 2-methylpentane recovery methods should consider multiple factors, including air and water quality, soil contamination risks, energy consumption, ecosystem impacts, and waste generation. By carefully evaluating these aspects, industries can select and implement recovery techniques that minimize environmental harm while maximizing resource efficiency.
Air quality is a primary concern in the recovery process of 2-methylpentane. Volatile organic compound (VOC) emissions are a significant risk, as 2-methylpentane is highly volatile. Different recovery methods may result in varying levels of VOC emissions, which can contribute to smog formation and negatively impact local air quality. Advanced recovery technologies, such as membrane separation or adsorption systems, generally have lower emission profiles compared to traditional distillation methods.
Water pollution is another critical factor to consider. Some recovery processes may generate wastewater containing trace amounts of 2-methylpentane or other organic compounds. Proper treatment and disposal of this wastewater are essential to prevent contamination of local water bodies. Closed-loop systems and advanced water treatment technologies can significantly reduce the risk of water pollution associated with the recovery process.
Soil contamination risks are generally lower compared to air and water impacts but should not be overlooked. Accidental spills or leaks during the recovery process could lead to soil pollution. Implementing robust containment measures and spill response protocols is crucial for minimizing these risks.
Energy consumption and greenhouse gas emissions associated with different recovery methods must also be evaluated. Some techniques, such as cryogenic distillation, may require significant energy inputs, potentially increasing the carbon footprint of the recovery process. On the other hand, more energy-efficient methods like pervaporation or adsorption may offer environmental benefits in terms of reduced energy consumption and associated emissions.
Biodiversity and ecosystem impacts should be assessed, particularly for recovery facilities located near sensitive habitats. While direct impacts on flora and fauna are typically limited, indirect effects through air and water emissions should be considered. Comprehensive environmental monitoring programs can help identify and mitigate any potential long-term impacts on local ecosystems.
Waste generation is another important aspect of the environmental assessment. Some recovery methods may produce solid waste, such as spent adsorbents or filter materials, which require proper disposal or regeneration. Evaluating the lifecycle of these materials and exploring recycling or regeneration options can help minimize the overall environmental footprint of the recovery process.
In conclusion, a thorough environmental impact assessment of 2-methylpentane recovery methods should consider multiple factors, including air and water quality, soil contamination risks, energy consumption, ecosystem impacts, and waste generation. By carefully evaluating these aspects, industries can select and implement recovery techniques that minimize environmental harm while maximizing resource efficiency.
Economic Feasibility Analysis
The economic feasibility analysis of recovering 2-methylpentane from waste streams is a critical aspect of implementing such a process in industrial settings. This analysis encompasses various factors that contribute to the overall cost-effectiveness and potential profitability of the recovery methods.
Capital expenditure (CAPEX) is a primary consideration, involving the initial investment required for equipment, infrastructure, and installation. For 2-methylpentane recovery, this may include distillation columns, extraction units, or membrane systems, depending on the chosen method. The scale of the operation and the purity requirements will significantly influence the CAPEX.
Operating expenses (OPEX) form another crucial component of the economic analysis. These ongoing costs include energy consumption, raw materials, labor, maintenance, and waste disposal. Energy costs are particularly significant in distillation processes, which are commonly used for hydrocarbon separation. Membrane-based methods may offer lower energy requirements but could incur higher replacement costs over time.
The market value of recovered 2-methylpentane is a key driver of economic feasibility. As a valuable solvent and chemical intermediate, its price fluctuations can significantly impact the recovery process's profitability. Current market trends and future demand projections should be carefully considered to assess long-term viability.
Recovery efficiency is another critical factor. Higher recovery rates generally improve economic feasibility, but this must be balanced against increased energy and equipment costs required to achieve higher purities. The trade-off between recovery rate and operational costs needs to be optimized for maximum economic benefit.
Environmental regulations and compliance costs must also be factored into the analysis. Stricter environmental policies may necessitate additional investments in emission control or waste treatment systems, impacting the overall economic feasibility.
The scale of operation plays a significant role in determining economic viability. Larger-scale operations often benefit from economies of scale, reducing per-unit production costs. However, this must be balanced against the available supply of waste streams containing 2-methylpentane and the market demand for the recovered product.
Potential revenue streams from by-products or co-products should not be overlooked. The recovery process may yield other valuable compounds that can be sold or utilized, enhancing the overall economic attractiveness of the project.
Lastly, a comprehensive economic feasibility analysis should include sensitivity analyses to account for uncertainties in key variables such as energy prices, raw material costs, and product market values. This approach helps in understanding the robustness of the economic model under various scenarios and aids in risk assessment for potential investors or stakeholders.
Capital expenditure (CAPEX) is a primary consideration, involving the initial investment required for equipment, infrastructure, and installation. For 2-methylpentane recovery, this may include distillation columns, extraction units, or membrane systems, depending on the chosen method. The scale of the operation and the purity requirements will significantly influence the CAPEX.
Operating expenses (OPEX) form another crucial component of the economic analysis. These ongoing costs include energy consumption, raw materials, labor, maintenance, and waste disposal. Energy costs are particularly significant in distillation processes, which are commonly used for hydrocarbon separation. Membrane-based methods may offer lower energy requirements but could incur higher replacement costs over time.
The market value of recovered 2-methylpentane is a key driver of economic feasibility. As a valuable solvent and chemical intermediate, its price fluctuations can significantly impact the recovery process's profitability. Current market trends and future demand projections should be carefully considered to assess long-term viability.
Recovery efficiency is another critical factor. Higher recovery rates generally improve economic feasibility, but this must be balanced against increased energy and equipment costs required to achieve higher purities. The trade-off between recovery rate and operational costs needs to be optimized for maximum economic benefit.
Environmental regulations and compliance costs must also be factored into the analysis. Stricter environmental policies may necessitate additional investments in emission control or waste treatment systems, impacting the overall economic feasibility.
The scale of operation plays a significant role in determining economic viability. Larger-scale operations often benefit from economies of scale, reducing per-unit production costs. However, this must be balanced against the available supply of waste streams containing 2-methylpentane and the market demand for the recovered product.
Potential revenue streams from by-products or co-products should not be overlooked. The recovery process may yield other valuable compounds that can be sold or utilized, enhancing the overall economic attractiveness of the project.
Lastly, a comprehensive economic feasibility analysis should include sensitivity analyses to account for uncertainties in key variables such as energy prices, raw material costs, and product market values. This approach helps in understanding the robustness of the economic model under various scenarios and aids in risk assessment for potential investors or stakeholders.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!