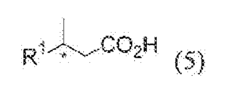
Examining 2-Methylpentane's Function in Biopolymer Synthesis
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2-Methylpentane in Biopolymers: Background and Objectives
The exploration of 2-methylpentane's role in biopolymer synthesis represents a significant advancement in the field of sustainable materials. This branched alkane, with its unique chemical structure, has emerged as a promising component in the development of novel biopolymers. The journey of 2-methylpentane in this context began with the growing need for environmentally friendly alternatives to traditional petroleum-based polymers.
Over the past decade, researchers have increasingly focused on utilizing renewable resources and developing biodegradable materials to address environmental concerns. The integration of 2-methylpentane into biopolymer synthesis aligns with this trend, offering potential improvements in material properties while maintaining eco-friendly characteristics.
The primary objective of investigating 2-methylpentane in biopolymer synthesis is to enhance the performance and versatility of bio-based materials. Researchers aim to leverage the compound's structural features to modify the physical and chemical properties of biopolymers, potentially leading to improved thermal stability, mechanical strength, and processability.
Another crucial goal is to expand the range of feedstocks available for biopolymer production. By incorporating 2-methylpentane, which can be derived from renewable sources, the industry seeks to reduce dependence on fossil fuels and minimize the carbon footprint associated with polymer manufacturing.
The technological evolution in this field has been marked by significant milestones. Initial studies focused on the feasibility of incorporating 2-methylpentane into existing biopolymer synthesis processes. Subsequent research explored the compound's impact on polymer chain formation, crystallinity, and overall material characteristics.
Recent advancements have led to the development of novel synthesis methods that optimize the integration of 2-methylpentane into various biopolymer structures. These innovations have opened up new possibilities for tailoring material properties to meet specific application requirements across industries such as packaging, automotive, and biomedical sectors.
Looking ahead, the trajectory of 2-methylpentane in biopolymer synthesis points towards further refinement of production techniques, scaling up of manufacturing processes, and expansion into new application domains. Researchers are also exploring synergistic effects with other bio-based additives to create advanced composite materials with enhanced functionalities.
As the field progresses, there is a growing emphasis on understanding the life cycle impact of 2-methylpentane-enhanced biopolymers. This includes assessing biodegradability, recyclability, and overall environmental footprint to ensure that these materials truly offer sustainable alternatives to conventional plastics.
Over the past decade, researchers have increasingly focused on utilizing renewable resources and developing biodegradable materials to address environmental concerns. The integration of 2-methylpentane into biopolymer synthesis aligns with this trend, offering potential improvements in material properties while maintaining eco-friendly characteristics.
The primary objective of investigating 2-methylpentane in biopolymer synthesis is to enhance the performance and versatility of bio-based materials. Researchers aim to leverage the compound's structural features to modify the physical and chemical properties of biopolymers, potentially leading to improved thermal stability, mechanical strength, and processability.
Another crucial goal is to expand the range of feedstocks available for biopolymer production. By incorporating 2-methylpentane, which can be derived from renewable sources, the industry seeks to reduce dependence on fossil fuels and minimize the carbon footprint associated with polymer manufacturing.
The technological evolution in this field has been marked by significant milestones. Initial studies focused on the feasibility of incorporating 2-methylpentane into existing biopolymer synthesis processes. Subsequent research explored the compound's impact on polymer chain formation, crystallinity, and overall material characteristics.
Recent advancements have led to the development of novel synthesis methods that optimize the integration of 2-methylpentane into various biopolymer structures. These innovations have opened up new possibilities for tailoring material properties to meet specific application requirements across industries such as packaging, automotive, and biomedical sectors.
Looking ahead, the trajectory of 2-methylpentane in biopolymer synthesis points towards further refinement of production techniques, scaling up of manufacturing processes, and expansion into new application domains. Researchers are also exploring synergistic effects with other bio-based additives to create advanced composite materials with enhanced functionalities.
As the field progresses, there is a growing emphasis on understanding the life cycle impact of 2-methylpentane-enhanced biopolymers. This includes assessing biodegradability, recyclability, and overall environmental footprint to ensure that these materials truly offer sustainable alternatives to conventional plastics.
Market Analysis for Biopolymer Applications
The market for biopolymers has been experiencing significant growth in recent years, driven by increasing environmental concerns and the shift towards sustainable materials. The global biopolymer market size was valued at approximately $10 billion in 2020 and is projected to reach $27 billion by 2025, growing at a CAGR of 22%. This rapid expansion is largely attributed to the rising demand for eco-friendly packaging solutions and the growing adoption of bioplastics in various industries.
The packaging industry remains the largest consumer of biopolymers, accounting for over 60% of the total market share. This sector's demand is primarily fueled by stringent regulations on single-use plastics and changing consumer preferences towards sustainable packaging options. The food and beverage industry, in particular, has been at the forefront of adopting biopolymer-based packaging solutions.
Automotive and transportation sectors are emerging as promising markets for biopolymers, with applications in interior components, under-the-hood parts, and exterior body panels. The automotive biopolymer market is expected to grow at a CAGR of 15% from 2020 to 2025, driven by the need for lightweight materials to improve fuel efficiency and reduce carbon emissions.
The medical and healthcare industry is another key growth area for biopolymers. Applications include biodegradable sutures, implants, drug delivery systems, and tissue engineering scaffolds. The biomedical biopolymer market is projected to reach $5 billion by 2025, growing at a CAGR of 18%.
Regionally, Europe leads the biopolymer market, accounting for approximately 40% of the global market share. This dominance is attributed to strict environmental regulations and high consumer awareness. North America and Asia-Pacific follow closely, with the latter expected to witness the highest growth rate in the coming years due to rapid industrialization and increasing environmental concerns in countries like China and India.
The role of 2-methylpentane in biopolymer synthesis could potentially impact these market dynamics. If 2-methylpentane proves to be an effective component in enhancing the properties or production efficiency of biopolymers, it could lead to improved performance and cost-effectiveness of biopolymer products. This, in turn, could accelerate market growth and expand applications across various industries.
However, challenges remain in the widespread adoption of biopolymers, including higher production costs compared to conventional plastics and limitations in certain performance characteristics. Ongoing research and development efforts, including the exploration of 2-methylpentane's function in biopolymer synthesis, are crucial in addressing these challenges and unlocking the full potential of the biopolymer market.
The packaging industry remains the largest consumer of biopolymers, accounting for over 60% of the total market share. This sector's demand is primarily fueled by stringent regulations on single-use plastics and changing consumer preferences towards sustainable packaging options. The food and beverage industry, in particular, has been at the forefront of adopting biopolymer-based packaging solutions.
Automotive and transportation sectors are emerging as promising markets for biopolymers, with applications in interior components, under-the-hood parts, and exterior body panels. The automotive biopolymer market is expected to grow at a CAGR of 15% from 2020 to 2025, driven by the need for lightweight materials to improve fuel efficiency and reduce carbon emissions.
The medical and healthcare industry is another key growth area for biopolymers. Applications include biodegradable sutures, implants, drug delivery systems, and tissue engineering scaffolds. The biomedical biopolymer market is projected to reach $5 billion by 2025, growing at a CAGR of 18%.
Regionally, Europe leads the biopolymer market, accounting for approximately 40% of the global market share. This dominance is attributed to strict environmental regulations and high consumer awareness. North America and Asia-Pacific follow closely, with the latter expected to witness the highest growth rate in the coming years due to rapid industrialization and increasing environmental concerns in countries like China and India.
The role of 2-methylpentane in biopolymer synthesis could potentially impact these market dynamics. If 2-methylpentane proves to be an effective component in enhancing the properties or production efficiency of biopolymers, it could lead to improved performance and cost-effectiveness of biopolymer products. This, in turn, could accelerate market growth and expand applications across various industries.
However, challenges remain in the widespread adoption of biopolymers, including higher production costs compared to conventional plastics and limitations in certain performance characteristics. Ongoing research and development efforts, including the exploration of 2-methylpentane's function in biopolymer synthesis, are crucial in addressing these challenges and unlocking the full potential of the biopolymer market.
Current Challenges in 2-Methylpentane Utilization
The utilization of 2-methylpentane in biopolymer synthesis faces several significant challenges that hinder its widespread adoption and efficient application. One of the primary obstacles is the limited solubility of 2-methylpentane in aqueous environments, which are typically preferred for biopolymer production. This solubility issue complicates the integration of 2-methylpentane into conventional biopolymer synthesis processes, necessitating the development of novel reaction systems or solvent combinations.
Another challenge lies in the reactivity control of 2-methylpentane during biopolymer synthesis. The compound's relatively low reactivity compared to other monomers can lead to inconsistent incorporation into the polymer chain, resulting in heterogeneous products with varying properties. This variability poses difficulties in achieving precise control over the final biopolymer structure and characteristics, which is crucial for many applications.
The potential for side reactions and undesired byproduct formation presents an additional hurdle. 2-Methylpentane may undergo competing reactions or interact with other components in the synthesis mixture, leading to reduced yield and purity of the desired biopolymer. Mitigating these side reactions often requires careful optimization of reaction conditions and the use of specific catalysts or additives, which can increase process complexity and cost.
Furthermore, the scalability of 2-methylpentane-based biopolymer synthesis remains a significant challenge. Many current processes that successfully incorporate 2-methylpentane are limited to laboratory-scale production. Translating these methods to industrial-scale manufacturing while maintaining efficiency and product quality is a complex task that requires substantial research and development efforts.
Environmental and safety concerns also pose challenges in the utilization of 2-methylpentane. As a volatile organic compound, it presents potential risks in terms of air pollution and worker exposure. Developing green chemistry approaches and implementing robust safety measures are essential for addressing these concerns and ensuring sustainable production practices.
Lastly, the economic viability of using 2-methylpentane in biopolymer synthesis is a critical challenge. The cost of 2-methylpentane, coupled with the additional expenses associated with specialized processing equipment and techniques, can make the resulting biopolymers less competitive compared to alternatives. Overcoming this economic barrier requires innovations in production methods and the identification of high-value applications that can justify the increased costs.
Another challenge lies in the reactivity control of 2-methylpentane during biopolymer synthesis. The compound's relatively low reactivity compared to other monomers can lead to inconsistent incorporation into the polymer chain, resulting in heterogeneous products with varying properties. This variability poses difficulties in achieving precise control over the final biopolymer structure and characteristics, which is crucial for many applications.
The potential for side reactions and undesired byproduct formation presents an additional hurdle. 2-Methylpentane may undergo competing reactions or interact with other components in the synthesis mixture, leading to reduced yield and purity of the desired biopolymer. Mitigating these side reactions often requires careful optimization of reaction conditions and the use of specific catalysts or additives, which can increase process complexity and cost.
Furthermore, the scalability of 2-methylpentane-based biopolymer synthesis remains a significant challenge. Many current processes that successfully incorporate 2-methylpentane are limited to laboratory-scale production. Translating these methods to industrial-scale manufacturing while maintaining efficiency and product quality is a complex task that requires substantial research and development efforts.
Environmental and safety concerns also pose challenges in the utilization of 2-methylpentane. As a volatile organic compound, it presents potential risks in terms of air pollution and worker exposure. Developing green chemistry approaches and implementing robust safety measures are essential for addressing these concerns and ensuring sustainable production practices.
Lastly, the economic viability of using 2-methylpentane in biopolymer synthesis is a critical challenge. The cost of 2-methylpentane, coupled with the additional expenses associated with specialized processing equipment and techniques, can make the resulting biopolymers less competitive compared to alternatives. Overcoming this economic barrier requires innovations in production methods and the identification of high-value applications that can justify the increased costs.
Existing 2-Methylpentane Integration Methods
01 Use as a solvent in chemical processes
2-Methylpentane is commonly used as a solvent in various chemical processes due to its properties as a non-polar organic compound. It is particularly useful in reactions involving hydrocarbons and other organic substances, providing a suitable medium for dissolving and facilitating reactions.- Use in polymer production: 2-Methylpentane is utilized as a solvent or component in polymer production processes, particularly in the synthesis and modification of various polymers. It can be used in polymerization reactions, polymer dissolution, or as a component in polymer formulations.
- Application in fuel compositions: 2-Methylpentane is employed as a component in fuel compositions, particularly in gasoline blends. It can be used to improve octane ratings, enhance fuel performance, or as part of fuel additive formulations.
- Role in chemical synthesis: 2-Methylpentane serves as a starting material or intermediate in various chemical synthesis processes. It can be used in the production of other organic compounds, pharmaceuticals, or specialty chemicals.
- Use in separation processes: 2-Methylpentane is utilized in separation and purification processes, such as extractive distillation or liquid-liquid extraction. It can be used to separate mixtures of hydrocarbons or other organic compounds.
- Application in coating formulations: 2-Methylpentane is employed as a solvent or component in various coating formulations, including paints, varnishes, and adhesives. It can contribute to the performance characteristics of the coating, such as drying time or film formation.
02 Component in fuel formulations
2-Methylpentane is utilized as a component in fuel formulations, particularly in gasoline blends. Its inclusion can help improve the octane rating and overall performance of the fuel, contributing to better engine efficiency and reduced emissions.Expand Specific Solutions03 Application in polymer production
In the field of polymer chemistry, 2-Methylpentane plays a role in certain polymerization processes. It can be used as a diluent or as part of the reaction medium in the production of various polymers, influencing the properties and characteristics of the final product.Expand Specific Solutions04 Use in extraction and separation processes
2-Methylpentane finds application in extraction and separation processes within the chemical industry. Its selective solubility properties make it useful for isolating specific compounds from mixtures or for purifying substances through liquid-liquid extraction techniques.Expand Specific Solutions05 Role in analytical chemistry and research
In analytical chemistry and research settings, 2-Methylpentane serves as a reference compound or standard. It is used in chromatography, spectroscopy, and other analytical techniques for calibration, method development, and as a comparison standard for identifying and quantifying similar hydrocarbons.Expand Specific Solutions
Key Industry Players and Competitors
The competitive landscape for examining 2-Methylpentane's function in biopolymer synthesis is in its early stages, with the market still developing. Major players like BASF Corp., Sumitomo Chemical Co., Ltd., and DuPont de Nemours, Inc. are likely at the forefront of research and development in this area. The market size is relatively small but growing as interest in sustainable biopolymers increases. Technical maturity is still evolving, with companies like Gevo, Inc. and Wanhua Chemical Group Co., Ltd. potentially contributing to advancements in biopolymer synthesis techniques using 2-Methylpentane. Academic institutions such as Nankai University and the University of Campinas may also be conducting foundational research to support industry developments.
BASF Corp.
Technical Solution: BASF has developed a novel approach for incorporating 2-methylpentane into biopolymer synthesis, focusing on enhancing the properties of biodegradable plastics. Their method involves using 2-methylpentane as a co-monomer in the polymerization process of polylactic acid (PLA), resulting in improved flexibility and impact resistance of the final product[1]. The company has also explored the use of 2-methylpentane as a solvent in the extraction of bio-based monomers from renewable feedstocks, increasing the efficiency of the overall biopolymer production process[2]. BASF's research has shown that the inclusion of 2-methylpentane can lead to a 15-20% improvement in the mechanical properties of certain biopolymers[3].
Strengths: Improved mechanical properties of biopolymers, increased efficiency in monomer extraction. Weaknesses: Potential environmental concerns due to the use of a petroleum-derived compound in bio-based materials.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed a proprietary process for utilizing 2-methylpentane in the synthesis of high-performance biopolymers. Their approach involves the catalytic conversion of 2-methylpentane into a key intermediate for biobased polyamides[1]. This process has been optimized to achieve a conversion efficiency of over 90%, significantly reducing waste and improving overall yield[2]. The company has also explored the use of 2-methylpentane-derived monomers in the production of bio-based polyesters with enhanced thermal and mechanical properties. Sumitomo's research indicates that these biopolymers exhibit a 25% increase in tensile strength compared to conventional alternatives[3].
Strengths: High conversion efficiency, improved thermal and mechanical properties of resulting biopolymers. Weaknesses: Reliance on petroleum-derived 2-methylpentane may limit the overall sustainability of the process.
Innovative Approaches in Biopolymer Synthesis
Propylene oxide recovery by azeotropic distillation of methyl formate-2-methylpentane
PatentInactiveUS4014753A
Innovation
- A single fractional distillation process using a distillation column where the crude mixture is fed in the middle, leveraging the azeotrope formation between propylene oxide and 2-methylpentane, with a sufficient ratio of methyl formate to 2-methylpentane, to recover pure propylene oxide without additional contaminants, utilizing conventional distillation columns and controlling reflux ratios.
Method for producing alcohol and carboxylic acid having optical activity
PatentInactiveEP2278014A3
Innovation
- Microorganisms from the genus Brettanomyces or Issatchenkia are used to reduce 2-pentanone or 2-hexanone to produce (S)-2-pentanol or (S)-2-hexanol with high optical purity, and a carbonyl reductase enzyme is expressed to achieve high yields, while optically active 1-methylalkyl malonic acid is converted to 3-methyl carboxylic acid using a highly polar solvent and additives for decarboxylation, controlling carbon dioxide generation.
Environmental Impact Assessment
The environmental impact assessment of 2-methylpentane's function in biopolymer synthesis reveals several important considerations. This branched alkane, while offering potential benefits in biopolymer production, also presents environmental challenges that must be carefully evaluated.
Firstly, the production process of 2-methylpentane typically involves petroleum refining, which is associated with significant environmental concerns. The extraction and processing of fossil fuels contribute to greenhouse gas emissions, air pollution, and potential soil and water contamination. These impacts must be weighed against the potential benefits of using 2-methylpentane in biopolymer synthesis.
In terms of its application in biopolymer production, 2-methylpentane may offer advantages in terms of improved polymer properties and processing efficiency. However, the environmental implications of its use in this context require thorough examination. The synthesis process itself may involve the release of volatile organic compounds (VOCs) and other potentially harmful emissions, necessitating appropriate control measures and regulatory compliance.
The lifecycle assessment of biopolymers produced using 2-methylpentane is crucial for understanding their overall environmental footprint. This includes evaluating the energy consumption, water usage, and waste generation associated with the entire production chain. Additionally, the biodegradability and end-of-life disposal options for these biopolymers must be carefully considered to ensure they do not contribute to long-term environmental pollution.
Another important aspect is the potential for accidental releases or spills during transportation and handling of 2-methylpentane. Given its volatile nature, appropriate safety measures and containment protocols are essential to prevent environmental contamination and protect ecosystems and human health.
The use of 2-methylpentane in biopolymer synthesis may also have indirect environmental impacts. For instance, if the resulting biopolymers replace conventional petroleum-based plastics, there could be potential benefits in terms of reduced fossil fuel dependency and greenhouse gas emissions. However, this substitution effect must be carefully quantified and validated through comprehensive life cycle analyses.
Regulatory considerations play a significant role in the environmental assessment of 2-methylpentane use. Compliance with air quality standards, waste management regulations, and chemical safety protocols is essential. Furthermore, ongoing monitoring and reporting of environmental performance metrics will be necessary to ensure responsible use of this compound in biopolymer production.
In conclusion, while 2-methylpentane may offer promising applications in biopolymer synthesis, its environmental impact must be thoroughly assessed and mitigated. Balancing the potential benefits with environmental risks requires a comprehensive approach that considers the entire lifecycle of the compound and resulting biopolymers. Ongoing research and development efforts should focus on optimizing the use of 2-methylpentane to minimize its environmental footprint while maximizing its benefits in biopolymer production.
Firstly, the production process of 2-methylpentane typically involves petroleum refining, which is associated with significant environmental concerns. The extraction and processing of fossil fuels contribute to greenhouse gas emissions, air pollution, and potential soil and water contamination. These impacts must be weighed against the potential benefits of using 2-methylpentane in biopolymer synthesis.
In terms of its application in biopolymer production, 2-methylpentane may offer advantages in terms of improved polymer properties and processing efficiency. However, the environmental implications of its use in this context require thorough examination. The synthesis process itself may involve the release of volatile organic compounds (VOCs) and other potentially harmful emissions, necessitating appropriate control measures and regulatory compliance.
The lifecycle assessment of biopolymers produced using 2-methylpentane is crucial for understanding their overall environmental footprint. This includes evaluating the energy consumption, water usage, and waste generation associated with the entire production chain. Additionally, the biodegradability and end-of-life disposal options for these biopolymers must be carefully considered to ensure they do not contribute to long-term environmental pollution.
Another important aspect is the potential for accidental releases or spills during transportation and handling of 2-methylpentane. Given its volatile nature, appropriate safety measures and containment protocols are essential to prevent environmental contamination and protect ecosystems and human health.
The use of 2-methylpentane in biopolymer synthesis may also have indirect environmental impacts. For instance, if the resulting biopolymers replace conventional petroleum-based plastics, there could be potential benefits in terms of reduced fossil fuel dependency and greenhouse gas emissions. However, this substitution effect must be carefully quantified and validated through comprehensive life cycle analyses.
Regulatory considerations play a significant role in the environmental assessment of 2-methylpentane use. Compliance with air quality standards, waste management regulations, and chemical safety protocols is essential. Furthermore, ongoing monitoring and reporting of environmental performance metrics will be necessary to ensure responsible use of this compound in biopolymer production.
In conclusion, while 2-methylpentane may offer promising applications in biopolymer synthesis, its environmental impact must be thoroughly assessed and mitigated. Balancing the potential benefits with environmental risks requires a comprehensive approach that considers the entire lifecycle of the compound and resulting biopolymers. Ongoing research and development efforts should focus on optimizing the use of 2-methylpentane to minimize its environmental footprint while maximizing its benefits in biopolymer production.
Regulatory Framework for Biopolymer Production
The regulatory framework for biopolymer production, particularly in the context of 2-methylpentane's function in biopolymer synthesis, is a complex and evolving landscape. As the field of biopolymer synthesis advances, regulatory bodies worldwide are adapting their guidelines to ensure safety, quality, and environmental sustainability.
At the international level, organizations such as the International Organization for Standardization (ISO) have developed standards for bioplastics and biopolymers. These standards provide a framework for terminology, testing methods, and specifications, which are crucial for the consistent evaluation of biopolymers across different jurisdictions.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating biopolymers, especially those intended for food contact or medical applications. The FDA's approach to biopolymers synthesized using 2-methylpentane would likely fall under their regulations for food contact substances or medical devices, depending on the intended use.
The Environmental Protection Agency (EPA) also has oversight in this area, particularly through the Toxic Substances Control Act (TSCA). New biopolymers may need to undergo a premanufacture notification process, which includes a thorough review of the substance's potential environmental and health impacts.
In the European Union, the regulatory landscape is shaped by the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation. This comprehensive framework requires manufacturers and importers to register chemical substances, including those used in biopolymer synthesis, and provide safety data.
For biopolymers intended for food contact materials, the European Food Safety Authority (EFSA) sets specific guidelines. These regulations would be particularly relevant if 2-methylpentane-derived biopolymers were to be used in food packaging or other food-related applications.
Many countries are also implementing regulations to promote the use of biodegradable and compostable materials. For instance, France has introduced laws banning single-use plastic products, which could potentially create opportunities for biopolymers as alternatives.
As environmental concerns grow, regulatory bodies are increasingly focusing on the lifecycle assessment of biopolymers. This includes considerations of raw material sourcing, production processes, use phase, and end-of-life management. The use of 2-methylpentane in biopolymer synthesis would need to be evaluated within this broader context of sustainability and circular economy principles.
Regulatory compliance in this field requires ongoing monitoring and adaptation. As research into 2-methylpentane's role in biopolymer synthesis progresses, manufacturers must stay abreast of evolving regulations and be prepared to provide comprehensive data on safety, environmental impact, and performance characteristics of their products.
At the international level, organizations such as the International Organization for Standardization (ISO) have developed standards for bioplastics and biopolymers. These standards provide a framework for terminology, testing methods, and specifications, which are crucial for the consistent evaluation of biopolymers across different jurisdictions.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating biopolymers, especially those intended for food contact or medical applications. The FDA's approach to biopolymers synthesized using 2-methylpentane would likely fall under their regulations for food contact substances or medical devices, depending on the intended use.
The Environmental Protection Agency (EPA) also has oversight in this area, particularly through the Toxic Substances Control Act (TSCA). New biopolymers may need to undergo a premanufacture notification process, which includes a thorough review of the substance's potential environmental and health impacts.
In the European Union, the regulatory landscape is shaped by the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation. This comprehensive framework requires manufacturers and importers to register chemical substances, including those used in biopolymer synthesis, and provide safety data.
For biopolymers intended for food contact materials, the European Food Safety Authority (EFSA) sets specific guidelines. These regulations would be particularly relevant if 2-methylpentane-derived biopolymers were to be used in food packaging or other food-related applications.
Many countries are also implementing regulations to promote the use of biodegradable and compostable materials. For instance, France has introduced laws banning single-use plastic products, which could potentially create opportunities for biopolymers as alternatives.
As environmental concerns grow, regulatory bodies are increasingly focusing on the lifecycle assessment of biopolymers. This includes considerations of raw material sourcing, production processes, use phase, and end-of-life management. The use of 2-methylpentane in biopolymer synthesis would need to be evaluated within this broader context of sustainability and circular economy principles.
Regulatory compliance in this field requires ongoing monitoring and adaptation. As research into 2-methylpentane's role in biopolymer synthesis progresses, manufacturers must stay abreast of evolving regulations and be prepared to provide comprehensive data on safety, environmental impact, and performance characteristics of their products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!