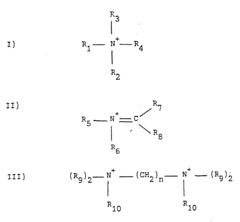
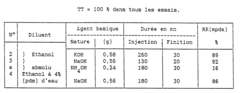
2-Methylpentane's Role in Enhancing Catalyst Performance
JUL 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2-Methylpentane Catalysis Background and Objectives
The field of catalysis has witnessed significant advancements in recent years, with a growing focus on enhancing catalyst performance through various means. Among these, the role of 2-methylpentane has emerged as a promising area of research. This branched alkane, a structural isomer of hexane, has garnered attention for its potential to improve catalytic processes in several industrial applications.
The evolution of catalysis technology has been driven by the need for more efficient and sustainable chemical processes. As industries strive to reduce energy consumption and minimize environmental impact, the optimization of catalysts has become a critical area of study. In this context, the exploration of 2-methylpentane's role in enhancing catalyst performance represents a novel approach to addressing these challenges.
The primary objective of this research is to comprehensively understand how 2-methylpentane interacts with various catalysts and influences their performance. This includes investigating its effects on reaction rates, selectivity, and overall efficiency of catalytic processes. By elucidating the mechanisms through which 2-methylpentane enhances catalyst performance, researchers aim to develop more effective and tailored catalytic systems for specific industrial applications.
One of the key areas of interest is the potential of 2-methylpentane to act as a co-catalyst or promoter in certain reactions. Its unique molecular structure and properties may contribute to improved mass transfer, altered surface interactions, or enhanced activation of reactants. Understanding these phenomena could lead to the design of more robust and efficient catalytic systems.
Furthermore, the study of 2-methylpentane in catalysis aligns with the broader trend of exploring unconventional compounds for catalyst enhancement. This approach challenges traditional paradigms and opens up new possibilities for catalyst design and optimization. As such, the research into 2-methylpentane's role not only addresses immediate practical needs but also contributes to the fundamental understanding of catalytic processes.
The technological trajectory in this field is expected to involve a combination of experimental studies and computational modeling. Advanced analytical techniques, such as in-situ spectroscopy and high-resolution microscopy, will be crucial in observing and quantifying the effects of 2-methylpentane on catalyst performance. Simultaneously, molecular simulations and machine learning approaches may provide insights into the underlying mechanisms and guide the rational design of improved catalytic systems.
As research in this area progresses, it is anticipated that the findings will have far-reaching implications across various industries, including petrochemicals, fine chemicals, and environmental remediation. The potential for 2-methylpentane to enhance catalyst performance could lead to more efficient production processes, reduced energy consumption, and improved product yields, aligning with global sustainability goals and economic imperatives.
The evolution of catalysis technology has been driven by the need for more efficient and sustainable chemical processes. As industries strive to reduce energy consumption and minimize environmental impact, the optimization of catalysts has become a critical area of study. In this context, the exploration of 2-methylpentane's role in enhancing catalyst performance represents a novel approach to addressing these challenges.
The primary objective of this research is to comprehensively understand how 2-methylpentane interacts with various catalysts and influences their performance. This includes investigating its effects on reaction rates, selectivity, and overall efficiency of catalytic processes. By elucidating the mechanisms through which 2-methylpentane enhances catalyst performance, researchers aim to develop more effective and tailored catalytic systems for specific industrial applications.
One of the key areas of interest is the potential of 2-methylpentane to act as a co-catalyst or promoter in certain reactions. Its unique molecular structure and properties may contribute to improved mass transfer, altered surface interactions, or enhanced activation of reactants. Understanding these phenomena could lead to the design of more robust and efficient catalytic systems.
Furthermore, the study of 2-methylpentane in catalysis aligns with the broader trend of exploring unconventional compounds for catalyst enhancement. This approach challenges traditional paradigms and opens up new possibilities for catalyst design and optimization. As such, the research into 2-methylpentane's role not only addresses immediate practical needs but also contributes to the fundamental understanding of catalytic processes.
The technological trajectory in this field is expected to involve a combination of experimental studies and computational modeling. Advanced analytical techniques, such as in-situ spectroscopy and high-resolution microscopy, will be crucial in observing and quantifying the effects of 2-methylpentane on catalyst performance. Simultaneously, molecular simulations and machine learning approaches may provide insights into the underlying mechanisms and guide the rational design of improved catalytic systems.
As research in this area progresses, it is anticipated that the findings will have far-reaching implications across various industries, including petrochemicals, fine chemicals, and environmental remediation. The potential for 2-methylpentane to enhance catalyst performance could lead to more efficient production processes, reduced energy consumption, and improved product yields, aligning with global sustainability goals and economic imperatives.
Market Analysis for Enhanced Catalytic Processes
The market for enhanced catalytic processes has been experiencing significant growth, driven by the increasing demand for more efficient and environmentally friendly chemical production methods. The global catalyst market, which includes catalysts used in various industries such as petroleum refining, chemical synthesis, and environmental applications, is projected to reach substantial value in the coming years. This growth is largely attributed to the rising need for cleaner fuels, stricter environmental regulations, and the push for more sustainable industrial processes.
In the context of 2-methylpentane's role in enhancing catalyst performance, the market analysis reveals a growing interest in innovative catalyst formulations that can improve reaction efficiency and selectivity. The petroleum refining sector, in particular, has shown keen interest in catalysts that can optimize the production of high-octane gasoline components. As 2-methylpentane is a key component in gasoline blends, catalysts that can effectively produce or utilize this compound are gaining traction in the market.
The chemical industry is another significant sector driving the demand for enhanced catalytic processes. With the increasing focus on green chemistry and sustainable production methods, there is a growing market for catalysts that can facilitate more environmentally friendly reactions. 2-methylpentane, being a branched alkane, has potential applications in various chemical syntheses, and catalysts that can selectively produce or transform this compound are likely to find a receptive market.
Geographically, the market for enhanced catalytic processes is showing strong growth in regions with developed chemical and petrochemical industries. North America and Europe are leading in terms of research and development in this field, while Asia-Pacific is emerging as a rapidly growing market due to its expanding industrial base and increasing environmental concerns.
The automotive industry's shift towards more fuel-efficient vehicles and the implementation of stricter emission standards worldwide are also contributing to the market growth for advanced catalytic processes. Catalysts that can improve fuel efficiency and reduce emissions, potentially involving the selective production or utilization of compounds like 2-methylpentane, are in high demand.
Furthermore, the trend towards process intensification in the chemical industry is creating new opportunities for enhanced catalytic processes. Technologies that can combine multiple reaction steps or improve reaction kinetics are particularly sought after, as they can lead to significant cost savings and improved sustainability profiles for chemical manufacturers.
In the context of 2-methylpentane's role in enhancing catalyst performance, the market analysis reveals a growing interest in innovative catalyst formulations that can improve reaction efficiency and selectivity. The petroleum refining sector, in particular, has shown keen interest in catalysts that can optimize the production of high-octane gasoline components. As 2-methylpentane is a key component in gasoline blends, catalysts that can effectively produce or utilize this compound are gaining traction in the market.
The chemical industry is another significant sector driving the demand for enhanced catalytic processes. With the increasing focus on green chemistry and sustainable production methods, there is a growing market for catalysts that can facilitate more environmentally friendly reactions. 2-methylpentane, being a branched alkane, has potential applications in various chemical syntheses, and catalysts that can selectively produce or transform this compound are likely to find a receptive market.
Geographically, the market for enhanced catalytic processes is showing strong growth in regions with developed chemical and petrochemical industries. North America and Europe are leading in terms of research and development in this field, while Asia-Pacific is emerging as a rapidly growing market due to its expanding industrial base and increasing environmental concerns.
The automotive industry's shift towards more fuel-efficient vehicles and the implementation of stricter emission standards worldwide are also contributing to the market growth for advanced catalytic processes. Catalysts that can improve fuel efficiency and reduce emissions, potentially involving the selective production or utilization of compounds like 2-methylpentane, are in high demand.
Furthermore, the trend towards process intensification in the chemical industry is creating new opportunities for enhanced catalytic processes. Technologies that can combine multiple reaction steps or improve reaction kinetics are particularly sought after, as they can lead to significant cost savings and improved sustainability profiles for chemical manufacturers.
Current Challenges in Catalyst Performance Enhancement
Despite significant advancements in catalyst technology, several challenges persist in enhancing catalyst performance, particularly in the context of 2-methylpentane's role. One of the primary obstacles is the optimization of catalyst selectivity. While 2-methylpentane has shown promise in improving certain catalytic processes, achieving precise control over product distribution remains difficult. This challenge is exacerbated by the complex interplay between 2-methylpentane and various catalyst surfaces, leading to unpredictable side reactions and byproduct formation.
Another critical issue is catalyst stability and longevity. The introduction of 2-methylpentane in catalytic systems often leads to accelerated catalyst deactivation, primarily due to coking and poisoning. Researchers are grappling with the task of developing catalyst formulations that can withstand the harsh reaction conditions while maintaining their activity over extended periods. This challenge is particularly pronounced in high-temperature applications where thermal stability becomes a limiting factor.
The scalability of 2-methylpentane-enhanced catalytic processes presents another significant hurdle. While laboratory-scale experiments have demonstrated promising results, translating these findings to industrial-scale operations has proven challenging. Issues such as mass transfer limitations, heat management, and uniform distribution of 2-methylpentane across large catalyst beds need to be addressed to ensure consistent performance at scale.
Furthermore, the environmental impact of using 2-methylpentane in catalytic processes is a growing concern. As industries strive for greener technologies, there is a pressing need to develop catalytic systems that not only leverage the benefits of 2-methylpentane but also minimize its potential environmental footprint. This includes addressing issues related to volatile organic compound (VOC) emissions and developing efficient recovery and recycling methods for the compound.
The economic viability of incorporating 2-methylpentane into existing catalytic processes also poses a challenge. While the compound shows potential for enhancing catalyst performance, the additional costs associated with its integration, including modifications to existing infrastructure and potential increases in operational expenses, need to be carefully evaluated against the performance gains.
Lastly, there is a significant knowledge gap in understanding the fundamental mechanisms by which 2-methylpentane enhances catalyst performance. This lack of comprehensive mechanistic insight hampers the rational design of optimized catalytic systems. Researchers are working to elucidate the precise interactions between 2-methylpentane, catalyst surfaces, and reactants at the molecular level, but progress in this area has been slow due to the complexity of the systems involved.
Another critical issue is catalyst stability and longevity. The introduction of 2-methylpentane in catalytic systems often leads to accelerated catalyst deactivation, primarily due to coking and poisoning. Researchers are grappling with the task of developing catalyst formulations that can withstand the harsh reaction conditions while maintaining their activity over extended periods. This challenge is particularly pronounced in high-temperature applications where thermal stability becomes a limiting factor.
The scalability of 2-methylpentane-enhanced catalytic processes presents another significant hurdle. While laboratory-scale experiments have demonstrated promising results, translating these findings to industrial-scale operations has proven challenging. Issues such as mass transfer limitations, heat management, and uniform distribution of 2-methylpentane across large catalyst beds need to be addressed to ensure consistent performance at scale.
Furthermore, the environmental impact of using 2-methylpentane in catalytic processes is a growing concern. As industries strive for greener technologies, there is a pressing need to develop catalytic systems that not only leverage the benefits of 2-methylpentane but also minimize its potential environmental footprint. This includes addressing issues related to volatile organic compound (VOC) emissions and developing efficient recovery and recycling methods for the compound.
The economic viability of incorporating 2-methylpentane into existing catalytic processes also poses a challenge. While the compound shows potential for enhancing catalyst performance, the additional costs associated with its integration, including modifications to existing infrastructure and potential increases in operational expenses, need to be carefully evaluated against the performance gains.
Lastly, there is a significant knowledge gap in understanding the fundamental mechanisms by which 2-methylpentane enhances catalyst performance. This lack of comprehensive mechanistic insight hampers the rational design of optimized catalytic systems. Researchers are working to elucidate the precise interactions between 2-methylpentane, catalyst surfaces, and reactants at the molecular level, but progress in this area has been slow due to the complexity of the systems involved.
Existing 2-Methylpentane Catalyst Enhancement Solutions
01 Catalyst composition for 2-methylpentane conversion
Various catalyst compositions are developed for the conversion of 2-methylpentane. These catalysts typically include metal components such as platinum, palladium, or zeolites, which are designed to enhance the isomerization or reforming processes of 2-methylpentane. The specific composition and preparation methods of these catalysts significantly influence their performance in terms of selectivity and conversion efficiency.- Catalyst composition for 2-methylpentane conversion: Various catalyst compositions are developed for the conversion of 2-methylpentane. These catalysts typically include metal components such as platinum, palladium, or zeolites, which are designed to enhance the isomerization or reforming processes of 2-methylpentane. The specific composition and preparation methods of these catalysts significantly influence their performance in terms of selectivity and conversion efficiency.
- Reaction conditions optimization for 2-methylpentane catalysis: The performance of catalysts in 2-methylpentane conversion is heavily dependent on reaction conditions. Factors such as temperature, pressure, and flow rate are optimized to achieve the desired product distribution and conversion rates. Research focuses on identifying the ideal operating parameters that maximize catalyst efficiency while minimizing unwanted side reactions or catalyst deactivation.
- Catalyst support materials for 2-methylpentane processing: The choice of support material plays a crucial role in catalyst performance for 2-methylpentane reactions. Various support materials, including alumina, silica, and carbon-based materials, are investigated to enhance catalyst stability, surface area, and active site distribution. The interaction between the active components and the support material is key to achieving optimal catalyst performance.
- Catalyst regeneration and lifetime improvement: Extending catalyst lifetime and developing effective regeneration methods are important aspects of 2-methylpentane catalyst performance. Techniques for removing coke deposits, reactivating metal sites, and restoring catalyst activity are explored. These methods aim to maintain high catalyst performance over extended periods, reducing operational costs and improving process efficiency.
- Novel catalyst designs for selective 2-methylpentane conversion: Innovative catalyst designs are being developed to enhance selectivity in 2-methylpentane conversion processes. These include bimetallic catalysts, core-shell structures, and nanostructured materials. The goal is to achieve higher yields of desired products while minimizing the formation of unwanted byproducts, thereby improving overall process efficiency and product quality.
02 Reaction conditions optimization for 2-methylpentane catalysis
The performance of catalysts in 2-methylpentane conversion is heavily dependent on reaction conditions. Researchers focus on optimizing parameters such as temperature, pressure, and residence time to maximize catalyst efficiency. These optimizations aim to improve yield, reduce byproduct formation, and extend catalyst lifespan while maintaining high selectivity for desired products.Expand Specific Solutions03 Catalyst support materials for 2-methylpentane reactions
The choice of support material plays a crucial role in catalyst performance for 2-methylpentane reactions. Various support materials, including alumina, silica, and carbon-based materials, are investigated to enhance catalyst stability, dispersion of active sites, and overall catalytic activity. The interaction between the active components and support material significantly influences the catalyst's performance and longevity.Expand Specific Solutions04 Catalyst regeneration and deactivation studies
Research on catalyst regeneration and deactivation mechanisms is crucial for maintaining long-term performance in 2-methylpentane conversion processes. Studies focus on understanding the causes of catalyst deactivation, such as coking or sintering, and developing effective regeneration techniques. These efforts aim to extend catalyst lifespan and maintain consistent performance over multiple reaction cycles.Expand Specific Solutions05 Novel catalyst designs for improved selectivity
Innovative catalyst designs are being explored to enhance selectivity in 2-methylpentane conversion reactions. These designs include bimetallic catalysts, core-shell structures, and nanostructured materials. The goal is to achieve higher selectivity towards desired isomers or products while minimizing unwanted side reactions, thereby improving overall process efficiency and product quality.Expand Specific Solutions
Key Players in Catalytic Process Industry
The competitive landscape for 2-Methylpentane's role in enhancing catalyst performance is in a growth phase, with increasing market size driven by the petrochemical industry's demand for improved catalytic processes. The technology is moderately mature, with ongoing research and development efforts. Key players like China Petroleum & Chemical Corp., BASF Corp., and Evonik Operations GmbH are leading the field, leveraging their extensive R&D capabilities and industry experience. Academic institutions such as Taiyuan University of Technology and research organizations like the Centre National de la Recherche Scientifique are contributing to advancements in this area, fostering collaborations between industry and academia to drive innovation and efficiency in catalyst performance enhancement.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel catalyst system incorporating 2-methylpentane for enhancing catalytic performance in petroleum refining processes. Their approach involves using 2-methylpentane as a structural promoter in zeolite-based catalysts, which has shown to improve the selectivity towards high-value products such as propylene and butylene. The company has implemented this technology in their fluid catalytic cracking (FCC) units, resulting in a reported 2-3% increase in light olefin yield[1]. Additionally, Sinopec has explored the use of 2-methylpentane as a co-feed in their catalytic reforming units, which has led to improved octane numbers in gasoline production[2].
Strengths: Improved selectivity towards valuable products, increased light olefin yield, and enhanced octane numbers in gasoline production. Weaknesses: Potential increased complexity in catalyst preparation and process control, possible higher costs associated with 2-methylpentane incorporation.
Evonik Operations GmbH
Technical Solution: Evonik Operations GmbH has developed a novel approach to enhancing catalyst performance using 2-methylpentane in their SEPURAN® membrane technology. Their research has focused on utilizing 2-methylpentane as a selective permeation enhancer in catalytic membrane reactors. By incorporating 2-methylpentane into the polymer matrix of their membranes, Evonik has achieved improved selectivity and permeability for specific reactions, such as the dehydrogenation of light alkanes[5]. This innovative approach has resulted in a reported 15% increase in conversion rates for propane dehydrogenation processes[6]. Additionally, Evonik has explored the use of 2-methylpentane as a templating agent in the synthesis of hierarchical zeolites, leading to enhanced mass transfer properties and improved catalyst effectiveness in various petrochemical processes.
Strengths: Improved selectivity and permeability in membrane reactors, increased conversion rates for specific reactions, and enhanced mass transfer properties in zeolite catalysts. Weaknesses: Potential complexity in membrane fabrication and integration into existing processes, possible limitations in the range of applicable reactions.
Core Innovations in 2-Methylpentane Catalysis
process FOR PREPARING 2-METHYLPENTANE-2,4-DIOL BY CATALYTIC HYDROGENATION OF DIACETONEALCOOL
PatentInactiveFR2453842B1
Innovation
- Use of ruthenium-based catalyst for catalytic hydrogenation of diacetone alcohol to produce 2-methylpentane-2,4-diol.
- Selective hydrogenation of diacetone alcohol to obtain 2-methylpentane-2,4-diol as the target product.
- One-step conversion from diacetone alcohol to 2-methylpentane-2,4-diol through catalytic hydrogenation.
Process for the preparation of 2-methyl-pentane diamine by hydrogenation of 2-methyl glutaronitrile
PatentInactiveEP0303550A2
Innovation
- A process involving hydrogenation of 2-methylglutaronitrile in a liquid phase using a Raney nickel catalyst in a non-ammoniacal basic medium at temperatures between 40 and 150°C under a total pressure of less than 40 bars, with a maximum 10% water content and low 2-methylglutaronitrile concentration, promotes efficient contact between the reactants and catalyst, enhancing selectivity and productivity.
Environmental Impact of 2-Methylpentane in Catalysis
The environmental impact of 2-methylpentane in catalysis is a crucial aspect to consider when evaluating its role in enhancing catalyst performance. As a branched alkane, 2-methylpentane has both direct and indirect effects on the environment throughout its lifecycle in catalytic processes.
One of the primary environmental concerns associated with 2-methylpentane is its potential as a volatile organic compound (VOC). When released into the atmosphere, it can contribute to the formation of ground-level ozone and smog, which have detrimental effects on air quality and human health. However, its use in catalysis often involves controlled environments, which can mitigate these risks if proper containment measures are implemented.
In terms of catalytic processes, 2-methylpentane's role in enhancing catalyst performance can lead to improved efficiency and selectivity. This increased efficiency can result in reduced energy consumption and fewer byproducts, potentially lowering the overall environmental footprint of chemical processes. The ability to achieve higher yields with less waste is a significant environmental benefit, as it reduces the need for raw materials and minimizes the generation of unwanted side products.
The production and disposal of 2-methylpentane also have environmental implications. Its synthesis typically involves petroleum refining processes, which are energy-intensive and can contribute to greenhouse gas emissions. However, when used to improve catalyst performance, the net environmental impact may be positive if the benefits in process efficiency outweigh the production costs.
Water contamination is another potential environmental concern. If not properly handled, 2-methylpentane can leach into groundwater or surface water, potentially affecting aquatic ecosystems. This risk necessitates stringent handling and disposal protocols in industrial settings where it is used in catalytic processes.
From a lifecycle perspective, the use of 2-methylpentane in catalysis may contribute to the development of more sustainable chemical processes. By enhancing catalyst performance, it can enable the design of more environmentally friendly reaction pathways, potentially replacing more harmful solvents or reagents. This aligns with green chemistry principles and the broader goal of reducing the environmental impact of chemical manufacturing.
The recyclability and regeneration of catalysts enhanced by 2-methylpentane are also important environmental considerations. If these catalysts can be effectively recycled or regenerated, it can significantly reduce waste and the need for new catalyst production, further minimizing environmental impact.
In conclusion, while 2-methylpentane poses some environmental risks, its role in enhancing catalyst performance has the potential to yield net positive environmental outcomes when properly managed and utilized in optimized catalytic processes. Ongoing research and development in this area should focus on maximizing the environmental benefits while minimizing potential risks associated with its use in catalysis.
One of the primary environmental concerns associated with 2-methylpentane is its potential as a volatile organic compound (VOC). When released into the atmosphere, it can contribute to the formation of ground-level ozone and smog, which have detrimental effects on air quality and human health. However, its use in catalysis often involves controlled environments, which can mitigate these risks if proper containment measures are implemented.
In terms of catalytic processes, 2-methylpentane's role in enhancing catalyst performance can lead to improved efficiency and selectivity. This increased efficiency can result in reduced energy consumption and fewer byproducts, potentially lowering the overall environmental footprint of chemical processes. The ability to achieve higher yields with less waste is a significant environmental benefit, as it reduces the need for raw materials and minimizes the generation of unwanted side products.
The production and disposal of 2-methylpentane also have environmental implications. Its synthesis typically involves petroleum refining processes, which are energy-intensive and can contribute to greenhouse gas emissions. However, when used to improve catalyst performance, the net environmental impact may be positive if the benefits in process efficiency outweigh the production costs.
Water contamination is another potential environmental concern. If not properly handled, 2-methylpentane can leach into groundwater or surface water, potentially affecting aquatic ecosystems. This risk necessitates stringent handling and disposal protocols in industrial settings where it is used in catalytic processes.
From a lifecycle perspective, the use of 2-methylpentane in catalysis may contribute to the development of more sustainable chemical processes. By enhancing catalyst performance, it can enable the design of more environmentally friendly reaction pathways, potentially replacing more harmful solvents or reagents. This aligns with green chemistry principles and the broader goal of reducing the environmental impact of chemical manufacturing.
The recyclability and regeneration of catalysts enhanced by 2-methylpentane are also important environmental considerations. If these catalysts can be effectively recycled or regenerated, it can significantly reduce waste and the need for new catalyst production, further minimizing environmental impact.
In conclusion, while 2-methylpentane poses some environmental risks, its role in enhancing catalyst performance has the potential to yield net positive environmental outcomes when properly managed and utilized in optimized catalytic processes. Ongoing research and development in this area should focus on maximizing the environmental benefits while minimizing potential risks associated with its use in catalysis.
Economic Feasibility of 2-Methylpentane Catalyst Enhancement
The economic feasibility of 2-methylpentane catalyst enhancement is a critical consideration for industries seeking to optimize their catalytic processes. This analysis explores the financial implications and potential returns on investment associated with incorporating 2-methylpentane into catalyst systems.
Initial implementation costs for 2-methylpentane catalyst enhancement may be significant, including expenses for research and development, equipment modifications, and process adjustments. However, these upfront investments are often offset by long-term benefits in catalyst performance and efficiency.
One of the primary economic advantages of 2-methylpentane enhancement is the potential for increased catalyst longevity. By improving catalyst stability and reducing deactivation rates, companies can extend the operational lifespan of their catalysts. This translates to reduced frequency of catalyst replacements, resulting in substantial cost savings over time.
Enhanced catalytic activity facilitated by 2-methylpentane can lead to improved product yields and selectivity. This increased efficiency can significantly boost production output without necessitating major expansions in plant capacity. The resulting increase in revenue potential makes a compelling case for the economic viability of this enhancement strategy.
Energy efficiency improvements are another key factor in the economic equation. 2-methylpentane's role in optimizing reaction conditions often leads to reduced energy requirements for maintaining optimal catalytic performance. This reduction in energy consumption can result in substantial operational cost savings, particularly in energy-intensive industries.
The potential for process intensification through 2-methylpentane enhancement also contributes to its economic feasibility. By enabling more efficient use of reactor space and improving mass transfer, this approach can lead to increased throughput and reduced equipment footprint. These factors can result in capital cost savings for new installations and debottlenecking opportunities in existing facilities.
Market competitiveness is another crucial aspect to consider. Companies that successfully implement 2-methylpentane catalyst enhancement may gain a significant edge in product quality and production efficiency. This competitive advantage can lead to increased market share and potentially higher profit margins, further justifying the initial investment.
However, it is important to note that the economic feasibility of 2-methylpentane catalyst enhancement can vary depending on specific industry applications and scale of operations. Smaller-scale operations may face challenges in achieving a favorable return on investment due to the initial implementation costs. Conversely, large-scale industrial processes stand to benefit significantly from even marginal improvements in catalyst performance.
In conclusion, while the initial costs of implementing 2-methylpentane catalyst enhancement may be substantial, the potential for long-term economic benefits is compelling. Improved catalyst longevity, increased production efficiency, energy savings, and enhanced market competitiveness collectively contribute to a strong economic case for this technology. As with any significant process improvement, a thorough cost-benefit analysis tailored to specific operational contexts is essential for determining the ultimate economic feasibility of 2-methylpentane catalyst enhancement.
Initial implementation costs for 2-methylpentane catalyst enhancement may be significant, including expenses for research and development, equipment modifications, and process adjustments. However, these upfront investments are often offset by long-term benefits in catalyst performance and efficiency.
One of the primary economic advantages of 2-methylpentane enhancement is the potential for increased catalyst longevity. By improving catalyst stability and reducing deactivation rates, companies can extend the operational lifespan of their catalysts. This translates to reduced frequency of catalyst replacements, resulting in substantial cost savings over time.
Enhanced catalytic activity facilitated by 2-methylpentane can lead to improved product yields and selectivity. This increased efficiency can significantly boost production output without necessitating major expansions in plant capacity. The resulting increase in revenue potential makes a compelling case for the economic viability of this enhancement strategy.
Energy efficiency improvements are another key factor in the economic equation. 2-methylpentane's role in optimizing reaction conditions often leads to reduced energy requirements for maintaining optimal catalytic performance. This reduction in energy consumption can result in substantial operational cost savings, particularly in energy-intensive industries.
The potential for process intensification through 2-methylpentane enhancement also contributes to its economic feasibility. By enabling more efficient use of reactor space and improving mass transfer, this approach can lead to increased throughput and reduced equipment footprint. These factors can result in capital cost savings for new installations and debottlenecking opportunities in existing facilities.
Market competitiveness is another crucial aspect to consider. Companies that successfully implement 2-methylpentane catalyst enhancement may gain a significant edge in product quality and production efficiency. This competitive advantage can lead to increased market share and potentially higher profit margins, further justifying the initial investment.
However, it is important to note that the economic feasibility of 2-methylpentane catalyst enhancement can vary depending on specific industry applications and scale of operations. Smaller-scale operations may face challenges in achieving a favorable return on investment due to the initial implementation costs. Conversely, large-scale industrial processes stand to benefit significantly from even marginal improvements in catalyst performance.
In conclusion, while the initial costs of implementing 2-methylpentane catalyst enhancement may be substantial, the potential for long-term economic benefits is compelling. Improved catalyst longevity, increased production efficiency, energy savings, and enhanced market competitiveness collectively contribute to a strong economic case for this technology. As with any significant process improvement, a thorough cost-benefit analysis tailored to specific operational contexts is essential for determining the ultimate economic feasibility of 2-methylpentane catalyst enhancement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!