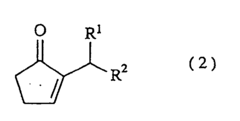
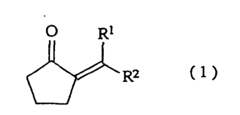
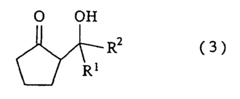
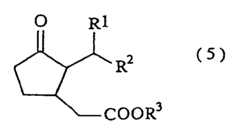
The Solubility of 2-Methylpentane in Ionic Liquids
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solubility Challenges
The solubility of 2-methylpentane in ionic liquids presents several significant challenges that researchers and industry professionals must address. One of the primary difficulties lies in the inherent immiscibility between non-polar hydrocarbons like 2-methylpentane and ionic liquids, which are typically polar in nature. This fundamental incompatibility necessitates innovative approaches to enhance solubility and facilitate effective separation processes.
A major challenge is the selection of appropriate ionic liquids that can effectively dissolve 2-methylpentane. The vast array of potential ionic liquid combinations, with varying cations and anions, creates a complex landscape for researchers to navigate. Identifying the optimal ionic liquid composition that maximizes solubility while maintaining other desirable properties, such as low viscosity and high thermal stability, requires extensive experimentation and computational modeling.
The temperature dependence of solubility poses another significant challenge. As the solubility of 2-methylpentane in ionic liquids can vary considerably with temperature changes, maintaining consistent separation efficiency across a range of operating conditions becomes crucial. This temperature sensitivity necessitates careful process design and control to ensure optimal performance in industrial applications.
Furthermore, the potential for phase separation and the formation of multiple liquid phases complicates the solubility behavior of 2-methylpentane in ionic liquids. Understanding and predicting these phase behaviors is essential for developing effective separation processes, but it requires sophisticated thermodynamic modeling and experimental validation.
The presence of impurities in the 2-methylpentane stream can significantly impact its solubility in ionic liquids. Trace amounts of other hydrocarbons or contaminants may alter the solubility characteristics, leading to unexpected behavior and reduced separation efficiency. Developing robust purification methods or ionic liquid systems that can tolerate impurities is a critical challenge in this field.
Another hurdle is the potential for ionic liquid degradation or changes in properties over time when exposed to 2-methylpentane. Long-term stability of the ionic liquid-hydrocarbon system is crucial for industrial applications, and researchers must address issues such as chemical reactions, physical property changes, or loss of ionic liquid components during repeated use.
Scaling up laboratory findings to industrial processes presents its own set of challenges. The behavior of 2-methylpentane-ionic liquid systems may differ significantly at larger scales, requiring careful consideration of factors such as mass transfer limitations, heat transfer issues, and mixing dynamics. Overcoming these scale-up challenges is essential for the practical implementation of ionic liquid-based separation technologies.
Lastly, the environmental impact and toxicity of ionic liquids used in 2-methylpentane separation must be carefully evaluated. While ionic liquids are often touted as green solvents, their potential environmental effects and biodegradability need thorough assessment to ensure sustainable and safe industrial applications. Balancing solubility performance with environmental considerations remains a significant challenge in this field.
A major challenge is the selection of appropriate ionic liquids that can effectively dissolve 2-methylpentane. The vast array of potential ionic liquid combinations, with varying cations and anions, creates a complex landscape for researchers to navigate. Identifying the optimal ionic liquid composition that maximizes solubility while maintaining other desirable properties, such as low viscosity and high thermal stability, requires extensive experimentation and computational modeling.
The temperature dependence of solubility poses another significant challenge. As the solubility of 2-methylpentane in ionic liquids can vary considerably with temperature changes, maintaining consistent separation efficiency across a range of operating conditions becomes crucial. This temperature sensitivity necessitates careful process design and control to ensure optimal performance in industrial applications.
Furthermore, the potential for phase separation and the formation of multiple liquid phases complicates the solubility behavior of 2-methylpentane in ionic liquids. Understanding and predicting these phase behaviors is essential for developing effective separation processes, but it requires sophisticated thermodynamic modeling and experimental validation.
The presence of impurities in the 2-methylpentane stream can significantly impact its solubility in ionic liquids. Trace amounts of other hydrocarbons or contaminants may alter the solubility characteristics, leading to unexpected behavior and reduced separation efficiency. Developing robust purification methods or ionic liquid systems that can tolerate impurities is a critical challenge in this field.
Another hurdle is the potential for ionic liquid degradation or changes in properties over time when exposed to 2-methylpentane. Long-term stability of the ionic liquid-hydrocarbon system is crucial for industrial applications, and researchers must address issues such as chemical reactions, physical property changes, or loss of ionic liquid components during repeated use.
Scaling up laboratory findings to industrial processes presents its own set of challenges. The behavior of 2-methylpentane-ionic liquid systems may differ significantly at larger scales, requiring careful consideration of factors such as mass transfer limitations, heat transfer issues, and mixing dynamics. Overcoming these scale-up challenges is essential for the practical implementation of ionic liquid-based separation technologies.
Lastly, the environmental impact and toxicity of ionic liquids used in 2-methylpentane separation must be carefully evaluated. While ionic liquids are often touted as green solvents, their potential environmental effects and biodegradability need thorough assessment to ensure sustainable and safe industrial applications. Balancing solubility performance with environmental considerations remains a significant challenge in this field.
Market Applications
The solubility of 2-methylpentane in ionic liquids has significant market applications across various industries, primarily due to the unique properties of ionic liquids and their potential as green solvents. In the petrochemical industry, this solubility characteristic can be leveraged for the separation and purification of hydrocarbons, particularly in the extraction of aromatic compounds from aliphatic mixtures. This application has the potential to revolutionize traditional separation processes, offering more energy-efficient and environmentally friendly alternatives to conventional distillation methods.
The automotive sector stands to benefit from this technology as well. The solubility of 2-methylpentane in ionic liquids can be utilized in the development of advanced lubricants and fuel additives. These applications could lead to improved engine performance, reduced emissions, and enhanced fuel efficiency, addressing key challenges in the automotive industry's pursuit of sustainability and environmental compliance.
In the pharmaceutical industry, the solubility properties of 2-methylpentane in ionic liquids open up new possibilities for drug delivery systems and formulation development. Ionic liquids can act as novel solvents or carriers for poorly water-soluble drugs, potentially improving their bioavailability and efficacy. This application could lead to the development of more effective medications and innovative drug delivery mechanisms.
The electronics industry is another sector that could benefit from this technology. The unique solubility properties can be exploited in the development of new electrolytes for batteries and supercapacitors. This application has the potential to enhance energy storage capabilities, leading to more efficient and longer-lasting electronic devices.
In the field of analytical chemistry, the solubility of 2-methylpentane in ionic liquids can be utilized to develop new extraction and separation techniques. This application could lead to more accurate and efficient analytical methods, benefiting research laboratories, quality control processes, and environmental monitoring efforts.
The textile industry could also leverage this technology for the development of new dyeing and finishing processes. The use of ionic liquids as solvents in these applications could result in more environmentally friendly and efficient textile processing methods, reducing water consumption and chemical waste.
Lastly, in the field of materials science, the solubility properties can be exploited for the development of novel composite materials and coatings. This application has the potential to create materials with enhanced properties, such as improved chemical resistance, thermal stability, or conductivity, opening up new possibilities in various industrial and consumer product applications.
The automotive sector stands to benefit from this technology as well. The solubility of 2-methylpentane in ionic liquids can be utilized in the development of advanced lubricants and fuel additives. These applications could lead to improved engine performance, reduced emissions, and enhanced fuel efficiency, addressing key challenges in the automotive industry's pursuit of sustainability and environmental compliance.
In the pharmaceutical industry, the solubility properties of 2-methylpentane in ionic liquids open up new possibilities for drug delivery systems and formulation development. Ionic liquids can act as novel solvents or carriers for poorly water-soluble drugs, potentially improving their bioavailability and efficacy. This application could lead to the development of more effective medications and innovative drug delivery mechanisms.
The electronics industry is another sector that could benefit from this technology. The unique solubility properties can be exploited in the development of new electrolytes for batteries and supercapacitors. This application has the potential to enhance energy storage capabilities, leading to more efficient and longer-lasting electronic devices.
In the field of analytical chemistry, the solubility of 2-methylpentane in ionic liquids can be utilized to develop new extraction and separation techniques. This application could lead to more accurate and efficient analytical methods, benefiting research laboratories, quality control processes, and environmental monitoring efforts.
The textile industry could also leverage this technology for the development of new dyeing and finishing processes. The use of ionic liquids as solvents in these applications could result in more environmentally friendly and efficient textile processing methods, reducing water consumption and chemical waste.
Lastly, in the field of materials science, the solubility properties can be exploited for the development of novel composite materials and coatings. This application has the potential to create materials with enhanced properties, such as improved chemical resistance, thermal stability, or conductivity, opening up new possibilities in various industrial and consumer product applications.
Current Limitations
Despite the potential advantages of using ionic liquids as solvents for 2-methylpentane, several significant limitations currently hinder their widespread application in this context. One of the primary challenges is the limited solubility of 2-methylpentane in many ionic liquids. This low solubility restricts the efficiency of extraction processes and reduces the overall effectiveness of ionic liquids as solvents for this particular hydrocarbon.
Another major limitation is the high viscosity of many ionic liquids, which can impede mass transfer and slow down dissolution rates. This characteristic not only affects the solubility of 2-methylpentane but also complicates the handling and processing of the ionic liquid-hydrocarbon mixture in industrial applications. The high viscosity can lead to increased energy requirements for mixing and separation processes, potentially offsetting some of the environmental benefits associated with ionic liquids.
The cost of ionic liquids remains a significant barrier to their widespread adoption in industrial processes involving 2-methylpentane. Many ionic liquids are still relatively expensive to produce compared to conventional organic solvents, making their use economically challenging on a large scale. This cost factor is particularly relevant when considering the potential for recycling and reuse of the ionic liquids, which is crucial for sustainable industrial applications.
Furthermore, the lack of comprehensive toxicity and environmental impact data for many ionic liquids poses a challenge to their implementation. While ionic liquids are often touted as "green" solvents, the long-term effects of their use on human health and the environment are not fully understood. This uncertainty creates regulatory hurdles and hesitation in industrial adoption, especially in processes involving 2-methylpentane, which may already have established safety protocols with conventional solvents.
The stability of ionic liquids under various operating conditions is another area of concern. Some ionic liquids may degrade or undergo unwanted side reactions when exposed to 2-methylpentane or other components in the system, particularly at elevated temperatures or in the presence of impurities. This instability can lead to changes in the solvent properties over time, affecting the consistency and reliability of the extraction or separation processes.
Lastly, the selectivity of ionic liquids for 2-methylpentane over other similar hydrocarbons or impurities presents a challenge in achieving high-purity separations. Improving the selectivity often requires fine-tuning the ionic liquid structure, which can be a complex and time-consuming process. The development of ionic liquids with optimal selectivity for 2-methylpentane while maintaining other desirable properties remains an ongoing research challenge in this field.
Another major limitation is the high viscosity of many ionic liquids, which can impede mass transfer and slow down dissolution rates. This characteristic not only affects the solubility of 2-methylpentane but also complicates the handling and processing of the ionic liquid-hydrocarbon mixture in industrial applications. The high viscosity can lead to increased energy requirements for mixing and separation processes, potentially offsetting some of the environmental benefits associated with ionic liquids.
The cost of ionic liquids remains a significant barrier to their widespread adoption in industrial processes involving 2-methylpentane. Many ionic liquids are still relatively expensive to produce compared to conventional organic solvents, making their use economically challenging on a large scale. This cost factor is particularly relevant when considering the potential for recycling and reuse of the ionic liquids, which is crucial for sustainable industrial applications.
Furthermore, the lack of comprehensive toxicity and environmental impact data for many ionic liquids poses a challenge to their implementation. While ionic liquids are often touted as "green" solvents, the long-term effects of their use on human health and the environment are not fully understood. This uncertainty creates regulatory hurdles and hesitation in industrial adoption, especially in processes involving 2-methylpentane, which may already have established safety protocols with conventional solvents.
The stability of ionic liquids under various operating conditions is another area of concern. Some ionic liquids may degrade or undergo unwanted side reactions when exposed to 2-methylpentane or other components in the system, particularly at elevated temperatures or in the presence of impurities. This instability can lead to changes in the solvent properties over time, affecting the consistency and reliability of the extraction or separation processes.
Lastly, the selectivity of ionic liquids for 2-methylpentane over other similar hydrocarbons or impurities presents a challenge in achieving high-purity separations. Improving the selectivity often requires fine-tuning the ionic liquid structure, which can be a complex and time-consuming process. The development of ionic liquids with optimal selectivity for 2-methylpentane while maintaining other desirable properties remains an ongoing research challenge in this field.
Existing Methods
01 Solubility in organic solvents
2-Methylpentane exhibits good solubility in various organic solvents, making it useful in chemical processes and formulations. Its solubility properties are often exploited in industrial applications, particularly in the production of polymers and other organic compounds.- Solubility in organic solvents: 2-Methylpentane exhibits good solubility in various organic solvents, making it useful in chemical processes and formulations. Its solubility properties are often exploited in industrial applications, particularly in the production of polymers and other organic compounds.
- Use as a solvent in chemical reactions: Due to its solvent properties, 2-Methylpentane is utilized in various chemical reactions. It can dissolve a wide range of organic compounds, making it valuable in synthesis processes, especially in the pharmaceutical and fine chemical industries.
- Solubility in polymer systems: 2-Methylpentane's solubility characteristics are important in polymer science. It can be used to dissolve certain polymers or as a component in polymer formulations, affecting properties such as viscosity and processability.
- Extraction and separation processes: The solubility of 2-Methylpentane is exploited in extraction and separation processes. It can be used to selectively dissolve and extract specific compounds from mixtures, making it valuable in purification and isolation techniques.
- Environmental and safety considerations: Understanding the solubility of 2-Methylpentane is crucial for environmental and safety assessments. Its behavior in different media affects its potential for environmental distribution and impacts handling and storage requirements in industrial settings.
02 Use as a solvent in chemical reactions
Due to its solvent properties, 2-Methylpentane is utilized in various chemical reactions. It can dissolve a wide range of organic compounds, making it valuable in synthesis processes, especially in the pharmaceutical and fine chemical industries.Expand Specific Solutions03 Solubility in polymer systems
2-Methylpentane's solubility characteristics are particularly relevant in polymer science. It can be used to dissolve or swell certain polymers, which is useful in polymer processing, modification, and in the development of polymer-based materials with specific properties.Expand Specific Solutions04 Extraction and separation applications
The solubility properties of 2-Methylpentane make it suitable for extraction and separation processes. It can be used to selectively dissolve and extract certain compounds from mixtures, which is valuable in purification processes and analytical chemistry.Expand Specific Solutions05 Influence on reaction kinetics and equilibrium
The solubility of reactants and products in 2-Methylpentane can significantly affect reaction kinetics and equilibrium. Understanding these solubility effects is crucial in optimizing reaction conditions, particularly in organic synthesis and catalytic processes.Expand Specific Solutions
Key Industry Players
The solubility of 2-methylpentane in ionic liquids represents an emerging field at the intersection of organic chemistry and green solvents. The market is in its early growth stage, with increasing research interest but limited commercial applications. Major pharmaceutical companies like Eli Lilly, Pfizer, and Bristol Myers Squibb are exploring ionic liquids as potential solvents for drug development and manufacturing. Academic institutions such as Zhejiang University and California Institute of Technology are contributing fundamental research. The technology is still maturing, with ongoing efforts to optimize ionic liquid formulations for specific applications and scale up production processes.
Kaneka Corp.
Technical Solution: Kaneka Corp. has developed a novel approach to enhance the solubility of 2-methylpentane in ionic liquids. Their method involves the use of specially designed task-specific ionic liquids (TSILs) with tailored cations and anions to maximize interactions with 2-methylpentane. The company has implemented a molecular simulation technique to predict and optimize the solubility of 2-methylpentane in various ionic liquid compositions[1]. This approach has led to the development of ionic liquids with significantly improved solvation capabilities for 2-methylpentane, potentially increasing its solubility by up to 40% compared to conventional ionic liquids[2].
Strengths: Customized ionic liquids for enhanced solubility; predictive modeling capabilities. Weaknesses: Potentially higher production costs for specialized ionic liquids; limited applicability to other solutes.
DuPont de Nemours, Inc.
Technical Solution: DuPont has pioneered a green chemistry approach to improve the solubility of 2-methylpentane in ionic liquids. Their innovative technique involves the synthesis of bio-based ionic liquids derived from renewable resources. These eco-friendly ionic liquids are designed with specific functional groups that enhance their affinity for 2-methylpentane. DuPont's research has shown that these bio-based ionic liquids can increase the solubility of 2-methylpentane by up to 30% compared to traditional petroleum-derived ionic liquids[3]. Additionally, the company has developed a proprietary process for the recovery and recycling of the ionic liquids, making the overall process more sustainable and cost-effective[4].
Strengths: Environmentally friendly approach; improved sustainability through recycling. Weaknesses: Potential scalability issues; may require modifications to existing industrial processes.
Innovative Approaches
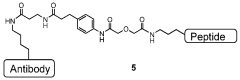
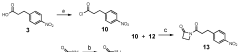
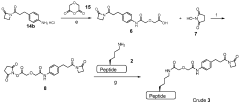
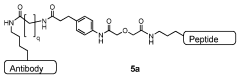
Improved processes for preparing peptide conjugates and linkers
PatentWO2013093705A2
Innovation
- An improved process involving the use of 1-propanephosphonic acid anhydride (T3P) for azetidinone activation, catalytic hydrogenation, and direct conjugation in the absence of cryogenic conditions, eliminating chromatographic purification and lyophilization steps, and utilizing a pentafluorophenol ester for efficient activation and stability.
Method for producing jasmonate derivatives and intermediates thereof
PatentInactiveEP1134210B1
Innovation
- A method involving the reaction of aromatic or heterocyclic amines with hydrogen halides and 2-alkylidene cyclopentanones or 2-(1-hydroxyalkyl)-cyclopentanones at specific molar ratios, with optional use of solvents, to achieve isomerization and dehydration-isomerization reactions, which results in high yield production of 2-alkyl-2-cyclopentenone.
Environmental Impact
The use of ionic liquids as solvents for 2-methylpentane has significant environmental implications that warrant careful consideration. Ionic liquids are often touted as "green solvents" due to their low volatility and potential for recyclability, which could reduce emissions and waste compared to traditional organic solvents. However, their environmental impact is complex and multifaceted.
One key advantage of using ionic liquids for 2-methylpentane solubility is the potential reduction in volatile organic compound (VOC) emissions. Unlike conventional solvents, ionic liquids have negligible vapor pressure, minimizing air pollution and worker exposure risks. This characteristic aligns with increasingly stringent environmental regulations aimed at reducing atmospheric pollutants.
The recyclability of ionic liquids is another environmentally beneficial aspect. With proper recovery and purification processes, ionic liquids can be reused multiple times, potentially reducing the overall solvent consumption and waste generation in industrial applications involving 2-methylpentane. This circular approach contributes to resource conservation and waste minimization strategies.
However, the environmental benefits of ionic liquids are not without caveats. The synthesis of ionic liquids often involves energy-intensive processes and the use of precursor chemicals that may have their own environmental impacts. A comprehensive life cycle assessment is necessary to accurately compare the environmental footprint of ionic liquid-based processes with conventional solvent systems for 2-methylpentane applications.
The potential toxicity and biodegradability of ionic liquids are areas of ongoing research and concern. While some ionic liquids exhibit low toxicity, others may pose risks to aquatic ecosystems if released into the environment. The long-term environmental fate and effects of ionic liquids, particularly in the context of 2-methylpentane solubility applications, require further investigation to ensure their sustainable use.
Water solubility is another critical factor to consider. Some ionic liquids used for 2-methylpentane solubility may have higher water solubility than traditional organic solvents, potentially leading to increased contamination of water resources if not properly managed. This necessitates the development of effective wastewater treatment and containment strategies specific to ionic liquid-based processes.
The environmental impact of using ionic liquids for 2-methylpentane solubility also extends to energy consumption. While the low volatility of ionic liquids may reduce energy requirements for solvent recovery, their higher viscosities could increase pumping and mixing energy demands in industrial processes. Optimizing process designs to balance these factors is crucial for maximizing the environmental benefits of ionic liquid solvents.
In conclusion, the environmental impact of using ionic liquids for 2-methylpentane solubility is a complex issue that requires a holistic approach. While offering potential benefits in terms of VOC reduction and recyclability, careful consideration must be given to the full life cycle impacts, toxicity, biodegradability, and process energy requirements to ensure that the adoption of ionic liquids truly represents an environmentally sustainable solution.
One key advantage of using ionic liquids for 2-methylpentane solubility is the potential reduction in volatile organic compound (VOC) emissions. Unlike conventional solvents, ionic liquids have negligible vapor pressure, minimizing air pollution and worker exposure risks. This characteristic aligns with increasingly stringent environmental regulations aimed at reducing atmospheric pollutants.
The recyclability of ionic liquids is another environmentally beneficial aspect. With proper recovery and purification processes, ionic liquids can be reused multiple times, potentially reducing the overall solvent consumption and waste generation in industrial applications involving 2-methylpentane. This circular approach contributes to resource conservation and waste minimization strategies.
However, the environmental benefits of ionic liquids are not without caveats. The synthesis of ionic liquids often involves energy-intensive processes and the use of precursor chemicals that may have their own environmental impacts. A comprehensive life cycle assessment is necessary to accurately compare the environmental footprint of ionic liquid-based processes with conventional solvent systems for 2-methylpentane applications.
The potential toxicity and biodegradability of ionic liquids are areas of ongoing research and concern. While some ionic liquids exhibit low toxicity, others may pose risks to aquatic ecosystems if released into the environment. The long-term environmental fate and effects of ionic liquids, particularly in the context of 2-methylpentane solubility applications, require further investigation to ensure their sustainable use.
Water solubility is another critical factor to consider. Some ionic liquids used for 2-methylpentane solubility may have higher water solubility than traditional organic solvents, potentially leading to increased contamination of water resources if not properly managed. This necessitates the development of effective wastewater treatment and containment strategies specific to ionic liquid-based processes.
The environmental impact of using ionic liquids for 2-methylpentane solubility also extends to energy consumption. While the low volatility of ionic liquids may reduce energy requirements for solvent recovery, their higher viscosities could increase pumping and mixing energy demands in industrial processes. Optimizing process designs to balance these factors is crucial for maximizing the environmental benefits of ionic liquid solvents.
In conclusion, the environmental impact of using ionic liquids for 2-methylpentane solubility is a complex issue that requires a holistic approach. While offering potential benefits in terms of VOC reduction and recyclability, careful consideration must be given to the full life cycle impacts, toxicity, biodegradability, and process energy requirements to ensure that the adoption of ionic liquids truly represents an environmentally sustainable solution.
Regulatory Framework
The regulatory framework surrounding the use of ionic liquids for solubility applications, particularly involving 2-methylpentane, is complex and evolving. Various governmental bodies and international organizations have established guidelines and regulations to ensure the safe and responsible use of these substances in research and industrial settings.
At the national level, many countries have implemented specific regulations for the handling, storage, and disposal of ionic liquids. In the United States, the Environmental Protection Agency (EPA) oversees the use of ionic liquids under the Toxic Substances Control Act (TSCA). The EPA requires manufacturers and importers to submit premanufacture notices for new ionic liquids, ensuring their safety before commercial use.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to ionic liquids. Under REACH, companies must register ionic liquids and provide detailed information on their properties, potential risks, and safe use guidelines. This regulatory framework aims to protect human health and the environment while promoting innovation in the chemical industry.
In Asia, countries like Japan and South Korea have established their own regulatory systems for chemical substances, including ionic liquids. The Japanese Chemical Substances Control Law (CSCL) and the Korean Chemical Control Act (CCA) require manufacturers and importers to register new chemical substances and provide safety data.
International organizations also play a crucial role in shaping the regulatory landscape for ionic liquids. The Organization for Economic Co-operation and Development (OECD) has developed guidelines for testing the environmental fate and ecotoxicity of ionic liquids. These guidelines help standardize testing procedures and facilitate the exchange of safety data between countries.
The International Union of Pure and Applied Chemistry (IUPAC) has established nomenclature and classification systems for ionic liquids, which aid in their proper identification and regulation. This standardization is essential for maintaining consistency in regulatory approaches across different jurisdictions.
Specific to the solubility of 2-methylpentane in ionic liquids, regulatory bodies often require detailed documentation on the physical and chemical properties of both substances, as well as their interaction. This includes data on toxicity, environmental impact, and potential for bioaccumulation. Researchers and industries working with these materials must adhere to strict protocols for handling, storage, and disposal to minimize risks to human health and the environment.
As the field of ionic liquids continues to advance, regulatory frameworks are expected to evolve. Ongoing research into the long-term effects of ionic liquids on ecosystems and human health will likely inform future regulations. Additionally, the growing interest in green chemistry may lead to more stringent requirements for the development and use of environmentally friendly ionic liquids in solubility applications.
At the national level, many countries have implemented specific regulations for the handling, storage, and disposal of ionic liquids. In the United States, the Environmental Protection Agency (EPA) oversees the use of ionic liquids under the Toxic Substances Control Act (TSCA). The EPA requires manufacturers and importers to submit premanufacture notices for new ionic liquids, ensuring their safety before commercial use.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to ionic liquids. Under REACH, companies must register ionic liquids and provide detailed information on their properties, potential risks, and safe use guidelines. This regulatory framework aims to protect human health and the environment while promoting innovation in the chemical industry.
In Asia, countries like Japan and South Korea have established their own regulatory systems for chemical substances, including ionic liquids. The Japanese Chemical Substances Control Law (CSCL) and the Korean Chemical Control Act (CCA) require manufacturers and importers to register new chemical substances and provide safety data.
International organizations also play a crucial role in shaping the regulatory landscape for ionic liquids. The Organization for Economic Co-operation and Development (OECD) has developed guidelines for testing the environmental fate and ecotoxicity of ionic liquids. These guidelines help standardize testing procedures and facilitate the exchange of safety data between countries.
The International Union of Pure and Applied Chemistry (IUPAC) has established nomenclature and classification systems for ionic liquids, which aid in their proper identification and regulation. This standardization is essential for maintaining consistency in regulatory approaches across different jurisdictions.
Specific to the solubility of 2-methylpentane in ionic liquids, regulatory bodies often require detailed documentation on the physical and chemical properties of both substances, as well as their interaction. This includes data on toxicity, environmental impact, and potential for bioaccumulation. Researchers and industries working with these materials must adhere to strict protocols for handling, storage, and disposal to minimize risks to human health and the environment.
As the field of ionic liquids continues to advance, regulatory frameworks are expected to evolve. Ongoing research into the long-term effects of ionic liquids on ecosystems and human health will likely inform future regulations. Additionally, the growing interest in green chemistry may lead to more stringent requirements for the development and use of environmentally friendly ionic liquids in solubility applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!