How 2-Methylpentane Supports Enhanced Electrochemical Reaction Processes
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2-Methylpentane Background and Objectives
2-Methylpentane, a branched alkane with the molecular formula C6H14, has emerged as a promising compound in the field of electrochemistry. Its unique structural properties and chemical characteristics have attracted significant attention from researchers and industry professionals alike, particularly in the context of enhancing electrochemical reaction processes.
The development of 2-methylpentane as a supporting agent in electrochemical reactions can be traced back to the early 2000s when scientists began exploring alternative solvents and additives to improve the efficiency and performance of various electrochemical systems. Initially, the focus was primarily on its potential as a low-viscosity, non-polar solvent for electrolyte solutions. However, as research progressed, it became evident that 2-methylpentane possessed additional properties that could significantly impact electrochemical reactions.
One of the key objectives in utilizing 2-methylpentane in electrochemical processes is to enhance the overall reaction kinetics. By modifying the electrode-electrolyte interface, 2-methylpentane has shown the potential to facilitate faster electron transfer rates and improve mass transport phenomena. This, in turn, can lead to increased reaction rates and higher overall efficiency in electrochemical systems.
Another important goal is to expand the electrochemical stability window of electrolyte solutions. The incorporation of 2-methylpentane has demonstrated the ability to widen the potential range within which electrochemical reactions can occur without significant solvent decomposition. This expanded stability window opens up new possibilities for electrochemical reactions that were previously limited by solvent breakdown.
Researchers are also exploring the use of 2-methylpentane to mitigate unwanted side reactions and improve the selectivity of desired electrochemical processes. By modifying the local environment at the electrode surface, 2-methylpentane may help suppress competing reactions and enhance the yield of target products.
Furthermore, there is growing interest in leveraging 2-methylpentane's properties to develop novel electrode materials and structures. The compound's interaction with various electrode surfaces could potentially lead to the creation of more efficient and durable electrodes for a wide range of applications, from energy storage devices to electrochemical sensors.
As the field of electrochemistry continues to evolve, understanding and optimizing the role of 2-methylpentane in supporting enhanced electrochemical reaction processes remains a critical area of research. The ongoing exploration of this compound's capabilities aligns with broader technological trends towards more efficient, sustainable, and versatile electrochemical systems across multiple industries and applications.
The development of 2-methylpentane as a supporting agent in electrochemical reactions can be traced back to the early 2000s when scientists began exploring alternative solvents and additives to improve the efficiency and performance of various electrochemical systems. Initially, the focus was primarily on its potential as a low-viscosity, non-polar solvent for electrolyte solutions. However, as research progressed, it became evident that 2-methylpentane possessed additional properties that could significantly impact electrochemical reactions.
One of the key objectives in utilizing 2-methylpentane in electrochemical processes is to enhance the overall reaction kinetics. By modifying the electrode-electrolyte interface, 2-methylpentane has shown the potential to facilitate faster electron transfer rates and improve mass transport phenomena. This, in turn, can lead to increased reaction rates and higher overall efficiency in electrochemical systems.
Another important goal is to expand the electrochemical stability window of electrolyte solutions. The incorporation of 2-methylpentane has demonstrated the ability to widen the potential range within which electrochemical reactions can occur without significant solvent decomposition. This expanded stability window opens up new possibilities for electrochemical reactions that were previously limited by solvent breakdown.
Researchers are also exploring the use of 2-methylpentane to mitigate unwanted side reactions and improve the selectivity of desired electrochemical processes. By modifying the local environment at the electrode surface, 2-methylpentane may help suppress competing reactions and enhance the yield of target products.
Furthermore, there is growing interest in leveraging 2-methylpentane's properties to develop novel electrode materials and structures. The compound's interaction with various electrode surfaces could potentially lead to the creation of more efficient and durable electrodes for a wide range of applications, from energy storage devices to electrochemical sensors.
As the field of electrochemistry continues to evolve, understanding and optimizing the role of 2-methylpentane in supporting enhanced electrochemical reaction processes remains a critical area of research. The ongoing exploration of this compound's capabilities aligns with broader technological trends towards more efficient, sustainable, and versatile electrochemical systems across multiple industries and applications.
Market Analysis for 2-Methylpentane in Electrochemistry
The market for 2-methylpentane in electrochemistry is experiencing significant growth, driven by the increasing demand for advanced energy storage and conversion technologies. As a key component in enhancing electrochemical reaction processes, 2-methylpentane has found applications in various sectors, including batteries, fuel cells, and electrochemical sensors.
In the battery market, 2-methylpentane has shown promise in improving the performance of lithium-ion batteries, which are widely used in consumer electronics, electric vehicles, and grid energy storage systems. The global lithium-ion battery market is projected to reach substantial growth in the coming years, with a compound annual growth rate (CAGR) exceeding industry averages. This growth is primarily fueled by the rapid adoption of electric vehicles and the increasing need for renewable energy storage solutions.
The fuel cell industry is another area where 2-methylpentane has demonstrated potential. As countries worldwide push for cleaner energy alternatives, the demand for fuel cells in transportation and stationary power generation is rising. The global fuel cell market is expected to expand significantly over the next decade, presenting opportunities for 2-methylpentane applications in enhancing electrochemical processes within these systems.
Electrochemical sensors represent another growing market segment where 2-methylpentane can play a crucial role. These sensors are used in various industries, including healthcare, environmental monitoring, and industrial process control. The global electrochemical sensor market is anticipated to show steady growth, driven by increasing awareness of environmental issues and the need for accurate and reliable sensing technologies.
The automotive sector is a key driver of demand for 2-methylpentane in electrochemistry applications. With the automotive industry's shift towards electrification, there is a growing need for advanced battery technologies and fuel cell systems. This transition is expected to create a substantial market for materials that can enhance electrochemical processes, such as 2-methylpentane.
Geographically, Asia-Pacific is expected to dominate the market for 2-methylpentane in electrochemistry applications, primarily due to the region's strong presence in battery manufacturing and electric vehicle production. North America and Europe are also significant markets, driven by investments in renewable energy and advanced transportation technologies.
However, the market faces challenges such as the volatility of raw material prices and the need for continuous research and development to improve the efficiency and cost-effectiveness of 2-methylpentane in electrochemical applications. Despite these challenges, the overall market outlook remains positive, with opportunities for growth in emerging applications and the potential for technological advancements to drive further adoption.
In the battery market, 2-methylpentane has shown promise in improving the performance of lithium-ion batteries, which are widely used in consumer electronics, electric vehicles, and grid energy storage systems. The global lithium-ion battery market is projected to reach substantial growth in the coming years, with a compound annual growth rate (CAGR) exceeding industry averages. This growth is primarily fueled by the rapid adoption of electric vehicles and the increasing need for renewable energy storage solutions.
The fuel cell industry is another area where 2-methylpentane has demonstrated potential. As countries worldwide push for cleaner energy alternatives, the demand for fuel cells in transportation and stationary power generation is rising. The global fuel cell market is expected to expand significantly over the next decade, presenting opportunities for 2-methylpentane applications in enhancing electrochemical processes within these systems.
Electrochemical sensors represent another growing market segment where 2-methylpentane can play a crucial role. These sensors are used in various industries, including healthcare, environmental monitoring, and industrial process control. The global electrochemical sensor market is anticipated to show steady growth, driven by increasing awareness of environmental issues and the need for accurate and reliable sensing technologies.
The automotive sector is a key driver of demand for 2-methylpentane in electrochemistry applications. With the automotive industry's shift towards electrification, there is a growing need for advanced battery technologies and fuel cell systems. This transition is expected to create a substantial market for materials that can enhance electrochemical processes, such as 2-methylpentane.
Geographically, Asia-Pacific is expected to dominate the market for 2-methylpentane in electrochemistry applications, primarily due to the region's strong presence in battery manufacturing and electric vehicle production. North America and Europe are also significant markets, driven by investments in renewable energy and advanced transportation technologies.
However, the market faces challenges such as the volatility of raw material prices and the need for continuous research and development to improve the efficiency and cost-effectiveness of 2-methylpentane in electrochemical applications. Despite these challenges, the overall market outlook remains positive, with opportunities for growth in emerging applications and the potential for technological advancements to drive further adoption.
Current Challenges in Electrochemical Reaction Processes
Electrochemical reaction processes have become increasingly important in various industries, from energy storage to chemical synthesis. However, several challenges persist in optimizing these processes for enhanced efficiency and performance. One of the primary obstacles is the limited understanding of the complex interactions between electrolytes, electrodes, and catalysts at the molecular level. This knowledge gap hinders the development of more effective and tailored electrochemical systems.
Another significant challenge is the stability and durability of electrodes and catalysts under harsh reaction conditions. Many electrochemical processes involve corrosive environments or high temperatures, which can lead to rapid degradation of materials and reduced performance over time. This issue is particularly pronounced in applications such as fuel cells and electrolyzers, where long-term stability is crucial for economic viability.
The scalability of electrochemical processes also presents a major hurdle. While many promising reactions have been demonstrated at the laboratory scale, translating these successes to industrial-scale operations often proves difficult. Factors such as mass transport limitations, heat management, and uniform current distribution become increasingly problematic as the scale of the system grows.
Energy efficiency remains a persistent challenge in electrochemical reactions. Overcoming activation barriers and minimizing side reactions that consume energy without contributing to the desired product formation are ongoing areas of research. This is particularly relevant in the context of renewable energy integration, where maximizing the efficiency of energy conversion and storage processes is paramount.
The selectivity of electrochemical reactions is another area of concern. Many processes suffer from poor product selectivity, leading to the formation of unwanted by-products and reduced overall efficiency. Developing catalysts and reaction conditions that can precisely control the reaction pathway is a key focus for researchers in the field.
Lastly, the environmental impact of electrochemical processes poses a challenge that must be addressed. While many of these technologies aim to provide more sustainable alternatives to traditional chemical processes, issues such as the use of rare or toxic materials in catalysts and the generation of waste products need careful consideration and mitigation strategies.
In the context of using 2-methylpentane to support enhanced electrochemical reaction processes, these challenges take on specific dimensions. The introduction of this organic compound into electrochemical systems may offer potential benefits, but it also raises questions about its stability, reactivity, and impact on electrode surfaces. Understanding how 2-methylpentane interacts with other components of the electrochemical cell and its role in potentially overcoming some of the aforementioned challenges is crucial for advancing this area of research.
Another significant challenge is the stability and durability of electrodes and catalysts under harsh reaction conditions. Many electrochemical processes involve corrosive environments or high temperatures, which can lead to rapid degradation of materials and reduced performance over time. This issue is particularly pronounced in applications such as fuel cells and electrolyzers, where long-term stability is crucial for economic viability.
The scalability of electrochemical processes also presents a major hurdle. While many promising reactions have been demonstrated at the laboratory scale, translating these successes to industrial-scale operations often proves difficult. Factors such as mass transport limitations, heat management, and uniform current distribution become increasingly problematic as the scale of the system grows.
Energy efficiency remains a persistent challenge in electrochemical reactions. Overcoming activation barriers and minimizing side reactions that consume energy without contributing to the desired product formation are ongoing areas of research. This is particularly relevant in the context of renewable energy integration, where maximizing the efficiency of energy conversion and storage processes is paramount.
The selectivity of electrochemical reactions is another area of concern. Many processes suffer from poor product selectivity, leading to the formation of unwanted by-products and reduced overall efficiency. Developing catalysts and reaction conditions that can precisely control the reaction pathway is a key focus for researchers in the field.
Lastly, the environmental impact of electrochemical processes poses a challenge that must be addressed. While many of these technologies aim to provide more sustainable alternatives to traditional chemical processes, issues such as the use of rare or toxic materials in catalysts and the generation of waste products need careful consideration and mitigation strategies.
In the context of using 2-methylpentane to support enhanced electrochemical reaction processes, these challenges take on specific dimensions. The introduction of this organic compound into electrochemical systems may offer potential benefits, but it also raises questions about its stability, reactivity, and impact on electrode surfaces. Understanding how 2-methylpentane interacts with other components of the electrochemical cell and its role in potentially overcoming some of the aforementioned challenges is crucial for advancing this area of research.
Existing 2-Methylpentane-Based Enhancement Solutions
01 Electrochemical reactions involving 2-methylpentane
Various electrochemical processes utilize 2-methylpentane as a reactant or solvent. These reactions may involve oxidation, reduction, or other transformations of the compound. The electrochemical reactions can be conducted in different cell configurations and under various conditions to achieve desired products or modifications of 2-methylpentane.- Electrochemical reactions involving 2-methylpentane: Various electrochemical processes utilize 2-methylpentane as a reactant or solvent. These reactions may involve oxidation, reduction, or other transformations of the compound. The electrochemical reactions can be conducted in different types of cells or reactors, potentially using catalysts or specific electrode materials to enhance selectivity or efficiency.
- Fuel cell applications using 2-methylpentane: 2-Methylpentane can be used in fuel cell systems, either as a fuel source or as a component in fuel mixtures. The compound may undergo electrochemical oxidation to generate electricity. Research focuses on optimizing electrode materials, catalysts, and operating conditions to improve fuel cell performance when using 2-methylpentane or related hydrocarbons.
- Electrochemical sensors for 2-methylpentane detection: Electrochemical sensors can be developed to detect and quantify 2-methylpentane in various environments. These sensors may utilize specific electrode materials or coatings that interact with the compound, producing a measurable electrical signal. Applications include environmental monitoring, quality control in industrial processes, and safety systems in areas where 2-methylpentane may be present.
- Electrochemical synthesis or modification of 2-methylpentane: Electrochemical methods can be employed to synthesize 2-methylpentane or modify its structure. This may involve electrochemical reduction of precursor compounds or selective functionalization of the molecule. Research in this area aims to develop more efficient and environmentally friendly routes for producing or derivatizing 2-methylpentane compared to traditional chemical methods.
- Electrochemical separation and purification processes: Electrochemical techniques can be used to separate or purify 2-methylpentane from mixtures or contaminated sources. These processes may involve selective electrochemical reactions, membrane separations, or electrochemically-driven extraction methods. The goal is to achieve high purity 2-methylpentane for various industrial or research applications.
02 Electrode materials for 2-methylpentane reactions
The choice of electrode materials plays a crucial role in electrochemical reactions involving 2-methylpentane. Various materials, including metals, alloys, and modified surfaces, are used to enhance reaction efficiency, selectivity, and product yield. The development of novel electrode materials aims to improve the overall performance of these electrochemical processes.Expand Specific Solutions03 Electrolyte systems for 2-methylpentane electrochemistry
Electrolyte composition and properties are essential factors in 2-methylpentane electrochemical reactions. Different electrolyte systems, including aqueous and non-aqueous solutions, are employed to facilitate ion transport and enhance reaction kinetics. The selection of appropriate electrolytes can significantly influence the reaction outcomes and overall process efficiency.Expand Specific Solutions04 Electrochemical cell designs for 2-methylpentane processing
Various electrochemical cell designs are utilized for reactions involving 2-methylpentane. These designs may include divided and undivided cells, flow cells, and specialized configurations to optimize reaction conditions. The cell design affects parameters such as mass transfer, current distribution, and product separation, which are crucial for efficient 2-methylpentane processing.Expand Specific Solutions05 Applications of 2-methylpentane electrochemical reactions
Electrochemical reactions involving 2-methylpentane find applications in various fields, including fuel cell technology, organic synthesis, and environmental remediation. These processes can be used for energy conversion, production of value-added chemicals, or treatment of 2-methylpentane-containing waste streams. The versatility of these electrochemical reactions contributes to their growing importance in industrial and research settings.Expand Specific Solutions
Key Players in 2-Methylpentane and Electrochemistry
The competitive landscape for 2-Methylpentane in enhanced electrochemical reaction processes is in an early development stage, with significant potential for growth. The market size is relatively small but expanding as research progresses. Technologically, it's still in the experimental phase, with academic institutions like Louisiana State University and California Institute of Technology leading fundamental research. Companies such as BASF Corp., Sinopec Shanghai Petrochemical Co., and Sinochem Lantian Co. are likely exploring applications, leveraging their expertise in chemical manufacturing. The technology's maturity is low, with most efforts focused on understanding mechanisms and optimizing performance rather than large-scale commercial implementation.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to enhance electrochemical reaction processes using 2-Methylpentane as a key component. Their method involves incorporating 2-Methylpentane into electrolyte solutions, which has been shown to improve the overall efficiency of electrochemical reactions. The company's research indicates that 2-Methylpentane acts as a co-solvent, enhancing the solubility of reactants and facilitating faster ion transport[1]. This results in increased reaction rates and improved electrode kinetics. BASF's technology also demonstrates a reduction in unwanted side reactions, leading to higher product selectivity and yield[2]. The company has successfully applied this approach in various electrochemical applications, including energy storage systems and electrosynthesis processes.
Strengths: Improved reaction efficiency, enhanced ion transport, and increased product selectivity. Weaknesses: Potential environmental concerns related to the use of volatile organic compounds, and the need for specialized handling and storage of 2-Methylpentane.
BP Chemicals Ltd.
Technical Solution: BP Chemicals Ltd. has developed an innovative electrochemical process that leverages the unique properties of 2-Methylpentane to enhance reaction efficiency. Their approach involves using 2-Methylpentane as a co-catalyst in electrochemical oxidation reactions, particularly in the production of fine chemicals and pharmaceuticals. BP's research shows that the addition of 2-Methylpentane to the reaction medium significantly improves the selectivity and yield of desired products[5]. The company's technology also demonstrates a reduction in energy consumption during the electrochemical process, as the presence of 2-Methylpentane lowers the activation energy required for the reactions to occur[6]. BP Chemicals has successfully implemented this technology in pilot-scale operations, showing promising results for industrial-scale applications.
Strengths: Improved product selectivity, reduced energy consumption, and potential for large-scale implementation. Weaknesses: Limited to specific types of electrochemical reactions and potential challenges in product separation.
Core Innovations in 2-Methylpentane Application
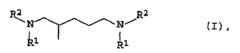
Process for the preparation of secondary or tertiary 2-methyl-1,5-pentanediamines
PatentInactiveEP0962447A3
Innovation
- A process involving the reaction of 2-methylglutarodinitrile with primary or secondary amines in the presence of an oxidic supported catalyst containing noble metals, where the catalyst is pre-treated with hydrogen at elevated temperatures and pressures, allowing for the production of secondary or tertiary 2-methyl-1,5-pentanediamines with improved yields and catalyst longevity.
Propylene oxide recovery by azeotropic distillation of methyl formate-2-methylpentane
PatentInactiveUS4014753A
Innovation
- A single fractional distillation process using a distillation column where the crude mixture is fed in the middle, leveraging the azeotrope formation between propylene oxide and 2-methylpentane, with a sufficient ratio of methyl formate to 2-methylpentane, to recover pure propylene oxide without additional contaminants, utilizing conventional distillation columns and controlling reflux ratios.
Environmental Impact of 2-Methylpentane Usage
The use of 2-methylpentane in enhanced electrochemical reaction processes raises important environmental considerations. As an organic solvent, 2-methylpentane has the potential to impact various aspects of the ecosystem if not properly managed. Its volatile nature contributes to the formation of ground-level ozone and smog when released into the atmosphere, potentially affecting air quality in urban and industrial areas.
In aquatic environments, 2-methylpentane can be toxic to marine life, particularly in cases of accidental spills or improper disposal. Its low water solubility allows it to form a film on water surfaces, potentially interfering with oxygen transfer and affecting aquatic organisms. Soil contamination is another concern, as 2-methylpentane can persist in soil and potentially leach into groundwater, posing risks to soil microorganisms and plant life.
From a lifecycle perspective, the production and use of 2-methylpentane contribute to carbon emissions, as it is derived from petroleum sources. This raises questions about its long-term sustainability in the context of global efforts to reduce greenhouse gas emissions and combat climate change. However, its role in enhancing electrochemical reactions may indirectly contribute to the development of more efficient energy storage and conversion technologies, potentially offsetting some of its environmental impacts.
Proper handling, storage, and disposal protocols are crucial to mitigate the environmental risks associated with 2-methylpentane. Implementing closed-loop systems in industrial processes can significantly reduce emissions and the potential for environmental contamination. Additionally, exploring bio-based alternatives or developing recycling methods for 2-methylpentane could further minimize its environmental footprint.
Regulatory frameworks play a vital role in managing the environmental impact of 2-methylpentane. Many countries have established guidelines for its use, storage, and disposal, often classifying it as a hazardous substance. Compliance with these regulations is essential for industries utilizing 2-methylpentane in electrochemical processes to ensure environmental protection and worker safety.
Research into greener alternatives and process optimizations is ongoing, aiming to reduce the reliance on potentially harmful solvents like 2-methylpentane. This includes exploring ionic liquids, supercritical fluids, and water-based systems as potential substitutes in electrochemical applications. Such innovations could lead to more environmentally friendly processes while maintaining or even improving reaction efficiencies.
In aquatic environments, 2-methylpentane can be toxic to marine life, particularly in cases of accidental spills or improper disposal. Its low water solubility allows it to form a film on water surfaces, potentially interfering with oxygen transfer and affecting aquatic organisms. Soil contamination is another concern, as 2-methylpentane can persist in soil and potentially leach into groundwater, posing risks to soil microorganisms and plant life.
From a lifecycle perspective, the production and use of 2-methylpentane contribute to carbon emissions, as it is derived from petroleum sources. This raises questions about its long-term sustainability in the context of global efforts to reduce greenhouse gas emissions and combat climate change. However, its role in enhancing electrochemical reactions may indirectly contribute to the development of more efficient energy storage and conversion technologies, potentially offsetting some of its environmental impacts.
Proper handling, storage, and disposal protocols are crucial to mitigate the environmental risks associated with 2-methylpentane. Implementing closed-loop systems in industrial processes can significantly reduce emissions and the potential for environmental contamination. Additionally, exploring bio-based alternatives or developing recycling methods for 2-methylpentane could further minimize its environmental footprint.
Regulatory frameworks play a vital role in managing the environmental impact of 2-methylpentane. Many countries have established guidelines for its use, storage, and disposal, often classifying it as a hazardous substance. Compliance with these regulations is essential for industries utilizing 2-methylpentane in electrochemical processes to ensure environmental protection and worker safety.
Research into greener alternatives and process optimizations is ongoing, aiming to reduce the reliance on potentially harmful solvents like 2-methylpentane. This includes exploring ionic liquids, supercritical fluids, and water-based systems as potential substitutes in electrochemical applications. Such innovations could lead to more environmentally friendly processes while maintaining or even improving reaction efficiencies.
Safety Considerations in 2-Methylpentane Handling
The safe handling of 2-methylpentane is crucial in its application for enhanced electrochemical reaction processes. As a highly flammable and volatile organic compound, 2-methylpentane poses significant risks that must be carefully managed in laboratory and industrial settings.
Proper storage is essential to prevent accidental release or ignition. 2-Methylpentane should be kept in tightly sealed containers in a cool, well-ventilated area away from sources of heat, sparks, or open flames. Storage areas should be equipped with appropriate fire suppression systems and explosion-proof electrical equipment.
Personal protective equipment (PPE) is vital when handling 2-methylpentane. Workers should wear chemical-resistant gloves, safety goggles, and protective clothing to prevent skin and eye contact. In cases where exposure to vapors is possible, respiratory protection may be necessary.
Ventilation is a critical safety measure when working with 2-methylpentane. All operations involving this compound should be conducted in a fume hood or well-ventilated area to prevent the accumulation of potentially explosive vapors. Local exhaust ventilation systems should be regularly maintained and tested for effectiveness.
Spill response procedures must be established and communicated to all personnel. In the event of a spill, the area should be immediately evacuated, and only trained personnel equipped with appropriate PPE should handle the cleanup. Absorbent materials specifically designed for organic solvents should be used, and contaminated materials must be disposed of as hazardous waste.
Fire safety is paramount when working with 2-methylpentane. Fire extinguishers suitable for flammable liquid fires (e.g., dry chemical, carbon dioxide, or foam) should be readily accessible. Employees must be trained in fire response procedures and the proper use of fire suppression equipment.
Electrical safety measures are essential to prevent ignition sources. All electrical equipment used in areas where 2-methylpentane is present should be explosion-proof and properly grounded. Regular inspections and maintenance of electrical systems are necessary to ensure ongoing safety.
Transportation of 2-methylpentane requires adherence to strict regulations. It must be transported in approved containers with proper labeling and documentation. Vehicles used for transportation should be equipped with appropriate safety features and placards indicating the presence of flammable materials.
Employee training is a critical component of safe 2-methylpentane handling. All personnel working with or around this compound should receive comprehensive training on its hazards, proper handling procedures, emergency response protocols, and the use of safety equipment.
Regular safety audits and risk assessments should be conducted to identify potential hazards and ensure that all safety measures remain effective. These assessments should include reviews of storage practices, handling procedures, emergency response plans, and employee training programs.
Proper storage is essential to prevent accidental release or ignition. 2-Methylpentane should be kept in tightly sealed containers in a cool, well-ventilated area away from sources of heat, sparks, or open flames. Storage areas should be equipped with appropriate fire suppression systems and explosion-proof electrical equipment.
Personal protective equipment (PPE) is vital when handling 2-methylpentane. Workers should wear chemical-resistant gloves, safety goggles, and protective clothing to prevent skin and eye contact. In cases where exposure to vapors is possible, respiratory protection may be necessary.
Ventilation is a critical safety measure when working with 2-methylpentane. All operations involving this compound should be conducted in a fume hood or well-ventilated area to prevent the accumulation of potentially explosive vapors. Local exhaust ventilation systems should be regularly maintained and tested for effectiveness.
Spill response procedures must be established and communicated to all personnel. In the event of a spill, the area should be immediately evacuated, and only trained personnel equipped with appropriate PPE should handle the cleanup. Absorbent materials specifically designed for organic solvents should be used, and contaminated materials must be disposed of as hazardous waste.
Fire safety is paramount when working with 2-methylpentane. Fire extinguishers suitable for flammable liquid fires (e.g., dry chemical, carbon dioxide, or foam) should be readily accessible. Employees must be trained in fire response procedures and the proper use of fire suppression equipment.
Electrical safety measures are essential to prevent ignition sources. All electrical equipment used in areas where 2-methylpentane is present should be explosion-proof and properly grounded. Regular inspections and maintenance of electrical systems are necessary to ensure ongoing safety.
Transportation of 2-methylpentane requires adherence to strict regulations. It must be transported in approved containers with proper labeling and documentation. Vehicles used for transportation should be equipped with appropriate safety features and placards indicating the presence of flammable materials.
Employee training is a critical component of safe 2-methylpentane handling. All personnel working with or around this compound should receive comprehensive training on its hazards, proper handling procedures, emergency response protocols, and the use of safety equipment.
Regular safety audits and risk assessments should be conducted to identify potential hazards and ensure that all safety measures remain effective. These assessments should include reviews of storage practices, handling procedures, emergency response plans, and employee training programs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!