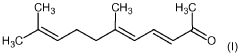
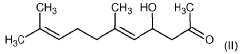
Application of 2-Methylpentane in Monitoring Chemical Reaction Kinetics
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2-Methylpentane Kinetics Background
The application of 2-methylpentane in monitoring chemical reaction kinetics represents a significant advancement in the field of analytical chemistry. This branched alkane, with its unique molecular structure and properties, has emerged as a valuable tool for studying the rates and mechanisms of chemical reactions.
2-Methylpentane, an isomer of hexane, possesses several characteristics that make it particularly suitable for kinetic studies. Its relatively low boiling point and high volatility allow for easy manipulation in gas-phase reactions. Additionally, its chemical stability under various conditions enables researchers to use it as a reliable probe molecule in a wide range of experimental setups.
The use of 2-methylpentane in reaction kinetics monitoring has evolved over time, driven by advancements in analytical techniques and instrumentation. Initially, its application was limited to simple gas-phase reactions, where it served as a model compound for studying hydrocarbon oxidation processes. However, as analytical methods improved, researchers began to explore its potential in more complex reaction systems.
One of the key advantages of using 2-methylpentane in kinetic studies is its ability to act as a sensitive indicator of reaction progress. By monitoring changes in its concentration or the formation of its reaction products, scientists can gain valuable insights into reaction rates, intermediates, and mechanisms. This has proven particularly useful in the study of combustion processes, atmospheric chemistry, and catalytic reactions.
The development of advanced spectroscopic techniques, such as gas chromatography-mass spectrometry (GC-MS) and Fourier-transform infrared spectroscopy (FTIR), has greatly enhanced the precision and sensitivity of 2-methylpentane-based kinetic measurements. These methods allow for real-time monitoring of reaction progress and the detection of trace amounts of intermediates and products.
In recent years, the application of 2-methylpentane in kinetic studies has expanded to include more diverse areas of chemistry. For instance, it has been used to investigate the kinetics of nanoparticle formation, the efficiency of photocatalytic processes, and the behavior of complex organic reactions. This versatility has made 2-methylpentane an invaluable tool in both fundamental research and industrial applications.
As the field of chemical kinetics continues to advance, the role of 2-methylpentane as a probe molecule is likely to grow. Ongoing research aims to further exploit its unique properties to develop new methodologies for studying increasingly complex reaction systems. The integration of 2-methylpentane-based techniques with emerging technologies, such as microfluidics and high-throughput screening, promises to open up new avenues for kinetic investigations in the future.
2-Methylpentane, an isomer of hexane, possesses several characteristics that make it particularly suitable for kinetic studies. Its relatively low boiling point and high volatility allow for easy manipulation in gas-phase reactions. Additionally, its chemical stability under various conditions enables researchers to use it as a reliable probe molecule in a wide range of experimental setups.
The use of 2-methylpentane in reaction kinetics monitoring has evolved over time, driven by advancements in analytical techniques and instrumentation. Initially, its application was limited to simple gas-phase reactions, where it served as a model compound for studying hydrocarbon oxidation processes. However, as analytical methods improved, researchers began to explore its potential in more complex reaction systems.
One of the key advantages of using 2-methylpentane in kinetic studies is its ability to act as a sensitive indicator of reaction progress. By monitoring changes in its concentration or the formation of its reaction products, scientists can gain valuable insights into reaction rates, intermediates, and mechanisms. This has proven particularly useful in the study of combustion processes, atmospheric chemistry, and catalytic reactions.
The development of advanced spectroscopic techniques, such as gas chromatography-mass spectrometry (GC-MS) and Fourier-transform infrared spectroscopy (FTIR), has greatly enhanced the precision and sensitivity of 2-methylpentane-based kinetic measurements. These methods allow for real-time monitoring of reaction progress and the detection of trace amounts of intermediates and products.
In recent years, the application of 2-methylpentane in kinetic studies has expanded to include more diverse areas of chemistry. For instance, it has been used to investigate the kinetics of nanoparticle formation, the efficiency of photocatalytic processes, and the behavior of complex organic reactions. This versatility has made 2-methylpentane an invaluable tool in both fundamental research and industrial applications.
As the field of chemical kinetics continues to advance, the role of 2-methylpentane as a probe molecule is likely to grow. Ongoing research aims to further exploit its unique properties to develop new methodologies for studying increasingly complex reaction systems. The integration of 2-methylpentane-based techniques with emerging technologies, such as microfluidics and high-throughput screening, promises to open up new avenues for kinetic investigations in the future.
Market Analysis for Reaction Monitoring
The market for reaction monitoring in chemical processes has been experiencing significant growth, driven by the increasing demand for efficient and precise control of chemical reactions across various industries. The global reaction monitoring market is expected to expand at a steady rate, with a particular focus on advanced technologies that offer real-time, in-situ monitoring capabilities.
One of the key factors driving this market growth is the rising emphasis on process optimization and quality control in chemical manufacturing. Industries such as pharmaceuticals, petrochemicals, and fine chemicals are increasingly adopting sophisticated reaction monitoring techniques to improve product yield, reduce waste, and ensure consistent product quality. This trend is further amplified by stringent regulatory requirements and the need for sustainable production practices.
The application of 2-Methylpentane in monitoring chemical reaction kinetics represents a niche but promising segment within the broader reaction monitoring market. As a solvent with unique properties, 2-Methylpentane offers potential advantages in certain reaction monitoring scenarios, particularly in organic synthesis and kinetic studies. Its low boiling point and chemical stability make it suitable for monitoring reactions that require precise temperature control and minimal interference from the monitoring medium.
The pharmaceutical industry is expected to be a major driver for the adoption of advanced reaction monitoring techniques, including those utilizing 2-Methylpentane. With the increasing complexity of drug molecules and the push towards continuous manufacturing processes, there is a growing need for sophisticated monitoring tools that can provide real-time insights into reaction progress and kinetics.
In the petrochemical sector, reaction monitoring plays a crucial role in optimizing refining processes and developing new catalysts. The use of 2-Methylpentane in this context could offer advantages in studying the kinetics of certain hydrocarbon reactions, potentially leading to more efficient and environmentally friendly production methods.
Academic and research institutions also represent a significant market segment for reaction monitoring technologies. The demand for advanced analytical tools in fundamental research and method development continues to grow, with a focus on techniques that can provide detailed mechanistic insights into chemical transformations.
The market for reaction monitoring solutions is characterized by a mix of established analytical instrument manufacturers and specialized technology providers. Companies offering integrated systems that combine multiple monitoring techniques, such as spectroscopy and chromatography, are likely to gain a competitive edge. Additionally, there is an increasing trend towards the development of miniaturized and portable monitoring devices, catering to the needs of on-site analysis and field research.
As the chemical industry continues to evolve towards more sustainable and efficient processes, the demand for innovative reaction monitoring solutions is expected to grow. The application of 2-Methylpentane in this field, while currently a niche area, has the potential to expand as researchers and industry professionals explore its unique properties for specific reaction monitoring challenges.
One of the key factors driving this market growth is the rising emphasis on process optimization and quality control in chemical manufacturing. Industries such as pharmaceuticals, petrochemicals, and fine chemicals are increasingly adopting sophisticated reaction monitoring techniques to improve product yield, reduce waste, and ensure consistent product quality. This trend is further amplified by stringent regulatory requirements and the need for sustainable production practices.
The application of 2-Methylpentane in monitoring chemical reaction kinetics represents a niche but promising segment within the broader reaction monitoring market. As a solvent with unique properties, 2-Methylpentane offers potential advantages in certain reaction monitoring scenarios, particularly in organic synthesis and kinetic studies. Its low boiling point and chemical stability make it suitable for monitoring reactions that require precise temperature control and minimal interference from the monitoring medium.
The pharmaceutical industry is expected to be a major driver for the adoption of advanced reaction monitoring techniques, including those utilizing 2-Methylpentane. With the increasing complexity of drug molecules and the push towards continuous manufacturing processes, there is a growing need for sophisticated monitoring tools that can provide real-time insights into reaction progress and kinetics.
In the petrochemical sector, reaction monitoring plays a crucial role in optimizing refining processes and developing new catalysts. The use of 2-Methylpentane in this context could offer advantages in studying the kinetics of certain hydrocarbon reactions, potentially leading to more efficient and environmentally friendly production methods.
Academic and research institutions also represent a significant market segment for reaction monitoring technologies. The demand for advanced analytical tools in fundamental research and method development continues to grow, with a focus on techniques that can provide detailed mechanistic insights into chemical transformations.
The market for reaction monitoring solutions is characterized by a mix of established analytical instrument manufacturers and specialized technology providers. Companies offering integrated systems that combine multiple monitoring techniques, such as spectroscopy and chromatography, are likely to gain a competitive edge. Additionally, there is an increasing trend towards the development of miniaturized and portable monitoring devices, catering to the needs of on-site analysis and field research.
As the chemical industry continues to evolve towards more sustainable and efficient processes, the demand for innovative reaction monitoring solutions is expected to grow. The application of 2-Methylpentane in this field, while currently a niche area, has the potential to expand as researchers and industry professionals explore its unique properties for specific reaction monitoring challenges.
Current Challenges in Kinetics Monitoring
The field of chemical reaction kinetics monitoring faces several significant challenges that hinder the accurate and efficient measurement of reaction rates and mechanisms. One of the primary obstacles is the limitation of current analytical techniques in providing real-time, in-situ measurements of reaction progress. Many traditional methods require sampling and ex-situ analysis, which can disrupt the reaction environment and introduce errors.
The complexity of multi-step reactions and the presence of intermediates pose another major challenge. Identifying and quantifying short-lived intermediate species is crucial for understanding reaction mechanisms, but these transient compounds are often difficult to detect and measure accurately. This complexity is further compounded in systems with multiple competing pathways or parallel reactions.
Temperature control and measurement present additional difficulties, especially for fast reactions or those with significant heat effects. Maintaining isothermal conditions throughout the reaction volume and accurately measuring local temperatures can be problematic, leading to uncertainties in kinetic data interpretation.
The influence of mass transfer limitations, particularly in heterogeneous systems or reactions involving gases and liquids, adds another layer of complexity to kinetics monitoring. Distinguishing between true kinetic effects and mass transfer phenomena requires sophisticated experimental design and data analysis techniques.
Sensitivity and selectivity of detection methods remain ongoing challenges, especially when dealing with trace amounts of reactants or products. Improving the lower limits of detection while maintaining high selectivity is crucial for studying slow reactions or those involving low-concentration species.
Data analysis and interpretation present their own set of challenges. The sheer volume of data generated by modern analytical techniques requires advanced computational methods and statistical tools to extract meaningful kinetic parameters. Fitting complex kinetic models to experimental data and discriminating between competing models can be particularly challenging.
Lastly, the development of robust, user-friendly, and cost-effective monitoring systems suitable for industrial applications remains an important goal. Many current high-precision techniques are limited to laboratory settings due to their complexity, cost, or sensitivity to environmental factors.
Addressing these challenges requires interdisciplinary approaches, combining advances in analytical chemistry, materials science, data science, and engineering. The application of novel materials like 2-methylpentane in kinetics monitoring may offer new avenues for overcoming some of these obstacles, potentially providing improved sensitivity, selectivity, or real-time monitoring capabilities.
The complexity of multi-step reactions and the presence of intermediates pose another major challenge. Identifying and quantifying short-lived intermediate species is crucial for understanding reaction mechanisms, but these transient compounds are often difficult to detect and measure accurately. This complexity is further compounded in systems with multiple competing pathways or parallel reactions.
Temperature control and measurement present additional difficulties, especially for fast reactions or those with significant heat effects. Maintaining isothermal conditions throughout the reaction volume and accurately measuring local temperatures can be problematic, leading to uncertainties in kinetic data interpretation.
The influence of mass transfer limitations, particularly in heterogeneous systems or reactions involving gases and liquids, adds another layer of complexity to kinetics monitoring. Distinguishing between true kinetic effects and mass transfer phenomena requires sophisticated experimental design and data analysis techniques.
Sensitivity and selectivity of detection methods remain ongoing challenges, especially when dealing with trace amounts of reactants or products. Improving the lower limits of detection while maintaining high selectivity is crucial for studying slow reactions or those involving low-concentration species.
Data analysis and interpretation present their own set of challenges. The sheer volume of data generated by modern analytical techniques requires advanced computational methods and statistical tools to extract meaningful kinetic parameters. Fitting complex kinetic models to experimental data and discriminating between competing models can be particularly challenging.
Lastly, the development of robust, user-friendly, and cost-effective monitoring systems suitable for industrial applications remains an important goal. Many current high-precision techniques are limited to laboratory settings due to their complexity, cost, or sensitivity to environmental factors.
Addressing these challenges requires interdisciplinary approaches, combining advances in analytical chemistry, materials science, data science, and engineering. The application of novel materials like 2-methylpentane in kinetics monitoring may offer new avenues for overcoming some of these obstacles, potentially providing improved sensitivity, selectivity, or real-time monitoring capabilities.
Existing 2-Methylpentane Monitoring Methods
01 Use in chemical synthesis and reactions
2-Methylpentane is utilized as a reagent or solvent in various chemical synthesis processes and reactions. It plays a role in organic chemistry applications, particularly in the production of other chemical compounds.- Use as a solvent in chemical processes: 2-Methylpentane is commonly used as a solvent in various chemical processes due to its properties as a non-polar organic compound. It is particularly useful in reactions involving hydrocarbons and other organic substances, providing a suitable medium for dissolving and processing materials.
- Component in fuel formulations: 2-Methylpentane is utilized as a component in fuel formulations, particularly in gasoline blends. Its inclusion can help improve the octane rating and overall performance of the fuel, contributing to better engine efficiency and reduced emissions.
- Application in polymer production: In the field of polymer chemistry, 2-Methylpentane finds application as a raw material or intermediate in the production of certain polymers and plastics. It can be used in polymerization processes or as a building block for more complex molecular structures.
- Use in extraction and separation processes: 2-Methylpentane is employed in extraction and separation processes, particularly in the petrochemical industry. Its physical and chemical properties make it suitable for selective extraction of specific compounds from mixtures or for use in chromatographic separations.
- Role in analytical chemistry and research: In analytical chemistry and research applications, 2-Methylpentane serves as a reference compound or standard. It is used in the calibration of instruments, development of analytical methods, and as a model compound for studying various chemical reactions and processes.
02 Application in polymer production
2-Methylpentane is employed in the production of polymers and copolymers. It can be used as a component in polymerization processes or as a solvent in polymer-related applications.Expand Specific Solutions03 Use as a fuel component
2-Methylpentane is utilized as a component in fuel formulations. It can be blended with other hydrocarbons to improve fuel properties or used in the production of high-performance fuels.Expand Specific Solutions04 Application in separation processes
2-Methylpentane is used in various separation processes, including extraction, distillation, and purification. It can serve as a solvent or an agent for separating different chemical compounds.Expand Specific Solutions05 Use in industrial cleaning and degreasing
2-Methylpentane finds applications in industrial cleaning and degreasing processes. It can be used as a solvent for removing oils, greases, and other contaminants from surfaces or equipment.Expand Specific Solutions
Key Players in Chemical Kinetics Industry
The application of 2-Methylpentane in monitoring chemical reaction kinetics is an emerging field within analytical chemistry. The market is in its early growth stage, with increasing interest from both academic and industrial sectors. While the market size is still relatively small, it is expected to expand as the technology matures. Companies like The Charles Stark Draper Laboratory, Life Technologies Corp., and Pacific Biosciences of California are at the forefront of developing and refining this technology. The technical maturity is moderate, with ongoing research to improve sensitivity, accuracy, and real-time monitoring capabilities. As more players enter the market and research progresses, we can expect to see advancements in instrumentation, data analysis, and application breadth in the coming years.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced techniques for monitoring chemical reaction kinetics using 2-methylpentane as a tracer molecule. Their approach involves using gas chromatography-mass spectrometry (GC-MS) to detect and quantify 2-methylpentane in real-time during petrochemical processes[1]. This method allows for precise tracking of reaction progress and kinetics in complex hydrocarbon mixtures. Sinopec has also implemented machine learning algorithms to analyze the 2-methylpentane concentration data, enabling predictive modeling of reaction rates and optimization of process conditions[3]. The company has successfully applied this technology in their refineries to improve the efficiency of catalytic cracking and reforming processes[5].
Strengths: Extensive experience in petrochemical processes, advanced analytical capabilities, and integration with existing refinery operations. Weaknesses: Potential limitations in non-petroleum applications and reliance on specialized equipment.
Swiss Federal Institute of Technology
Technical Solution: The Swiss Federal Institute of Technology (ETH Zurich) has pioneered the use of 2-methylpentane in monitoring chemical reaction kinetics through innovative spectroscopic techniques. Their research team has developed a novel approach using time-resolved infrared spectroscopy to track the concentration of 2-methylpentane during reactions[2]. This method allows for high-resolution, real-time monitoring of reaction progress without the need for sample extraction. ETH Zurich has also combined this technique with microfluidic devices, enabling the study of fast reactions and providing insights into reaction mechanisms at the molecular level[4]. Additionally, they have explored the use of 2-methylpentane as a probe molecule for investigating solvent effects on reaction kinetics, contributing to a deeper understanding of reaction environments[6].
Strengths: Cutting-edge spectroscopic techniques, expertise in fundamental reaction kinetics, and integration with microfluidic technologies. Weaknesses: Potential scalability issues for industrial applications and limited focus on large-scale process monitoring.
Innovations in 2-Methylpentane Detection
Propylene oxide recovery by azeotropic distillation of methyl formate-2-methylpentane
PatentInactiveUS4014753A
Innovation
- A single fractional distillation process using a distillation column where the crude mixture is fed in the middle, leveraging the azeotrope formation between propylene oxide and 2-methylpentane, with a sufficient ratio of methyl formate to 2-methylpentane, to recover pure propylene oxide without additional contaminants, utilizing conventional distillation columns and controlling reflux ratios.
Process of making pseudoionone and hydroxy pseudoionone in aqueous mixtures comprising citral and acetone, comprising adding first and second amounts of hydroxide
PatentWO2020099452A1
Innovation
- A process involving the preparation of an initial aqueous mixture with acetone, citral, and hydroxide, followed by a reaction to form pseudoionone, hydroxy pseudoionone, and 4-hydroxy-4-methylpentan-2-one, with a subsequent addition of hydroxide to enhance pseudoionone formation, utilizing a continuous apparatus with specific reactor configurations to optimize reaction conditions.
Environmental Impact Assessment
The application of 2-Methylpentane in monitoring chemical reaction kinetics necessitates a thorough environmental impact assessment to ensure its safe and sustainable use. This assessment primarily focuses on the potential effects of 2-Methylpentane on air quality, water resources, and soil composition.
In terms of air quality, 2-Methylpentane is a volatile organic compound (VOC) that can contribute to the formation of ground-level ozone and smog when released into the atmosphere. Its use in chemical reaction monitoring may lead to increased emissions, particularly in laboratory or industrial settings. These emissions could potentially exacerbate air pollution issues in urban areas or regions with existing air quality concerns.
Water contamination is another critical aspect to consider. 2-Methylpentane has low water solubility but can still pose risks to aquatic ecosystems if released in significant quantities. Accidental spills or improper disposal of waste containing this compound could lead to surface water or groundwater contamination. The potential for bioaccumulation in aquatic organisms and subsequent impacts on the food chain should be thoroughly evaluated.
Soil contamination is a third area of concern. Although 2-Methylpentane is expected to have high mobility in soil due to its physical properties, prolonged exposure or large-scale releases could potentially affect soil microorganisms and plant life. The compound's persistence in soil and its potential for leaching into groundwater should be carefully assessed.
The environmental fate of 2-Methylpentane is an important consideration. Its relatively high vapor pressure suggests that it will primarily partition to the air when released into the environment. However, the potential for long-range transport and deposition in remote areas should be evaluated, particularly in the context of global atmospheric circulation patterns.
Biodegradation pathways and rates for 2-Methylpentane in various environmental compartments (air, water, and soil) should be studied to understand its persistence and potential for accumulation. Additionally, the formation of breakdown products and their respective environmental impacts must be considered in the overall assessment.
Mitigation strategies and best practices for handling, storing, and disposing of 2-Methylpentane should be developed based on the findings of the environmental impact assessment. These may include engineering controls to minimize emissions, proper containment measures, and treatment protocols for contaminated water or soil.
Lastly, the assessment should consider the broader lifecycle impacts of 2-Methylpentane use in chemical reaction kinetics monitoring. This includes the environmental footprint of its production, transportation, and eventual disposal or recycling. A comprehensive understanding of these factors will enable more informed decision-making regarding the application of 2-Methylpentane in scientific and industrial processes, balancing its benefits in reaction monitoring against potential environmental risks.
In terms of air quality, 2-Methylpentane is a volatile organic compound (VOC) that can contribute to the formation of ground-level ozone and smog when released into the atmosphere. Its use in chemical reaction monitoring may lead to increased emissions, particularly in laboratory or industrial settings. These emissions could potentially exacerbate air pollution issues in urban areas or regions with existing air quality concerns.
Water contamination is another critical aspect to consider. 2-Methylpentane has low water solubility but can still pose risks to aquatic ecosystems if released in significant quantities. Accidental spills or improper disposal of waste containing this compound could lead to surface water or groundwater contamination. The potential for bioaccumulation in aquatic organisms and subsequent impacts on the food chain should be thoroughly evaluated.
Soil contamination is a third area of concern. Although 2-Methylpentane is expected to have high mobility in soil due to its physical properties, prolonged exposure or large-scale releases could potentially affect soil microorganisms and plant life. The compound's persistence in soil and its potential for leaching into groundwater should be carefully assessed.
The environmental fate of 2-Methylpentane is an important consideration. Its relatively high vapor pressure suggests that it will primarily partition to the air when released into the environment. However, the potential for long-range transport and deposition in remote areas should be evaluated, particularly in the context of global atmospheric circulation patterns.
Biodegradation pathways and rates for 2-Methylpentane in various environmental compartments (air, water, and soil) should be studied to understand its persistence and potential for accumulation. Additionally, the formation of breakdown products and their respective environmental impacts must be considered in the overall assessment.
Mitigation strategies and best practices for handling, storing, and disposing of 2-Methylpentane should be developed based on the findings of the environmental impact assessment. These may include engineering controls to minimize emissions, proper containment measures, and treatment protocols for contaminated water or soil.
Lastly, the assessment should consider the broader lifecycle impacts of 2-Methylpentane use in chemical reaction kinetics monitoring. This includes the environmental footprint of its production, transportation, and eventual disposal or recycling. A comprehensive understanding of these factors will enable more informed decision-making regarding the application of 2-Methylpentane in scientific and industrial processes, balancing its benefits in reaction monitoring against potential environmental risks.
Safety Protocols and Regulations
The use of 2-Methylpentane in monitoring chemical reaction kinetics necessitates strict adherence to safety protocols and regulations. These guidelines are crucial for protecting researchers, laboratory personnel, and the environment from potential hazards associated with this volatile organic compound.
Proper handling and storage of 2-Methylpentane are paramount. The chemical should be kept in tightly sealed containers in a cool, well-ventilated area away from sources of ignition. Due to its high flammability, fire safety measures must be in place, including readily accessible fire extinguishers and sprinkler systems. Laboratories should be equipped with fume hoods or local exhaust ventilation systems to minimize exposure to vapors.
Personal protective equipment (PPE) is essential when working with 2-Methylpentane. This includes chemical-resistant gloves, safety goggles, and lab coats. In cases where exposure limits may be exceeded, respiratory protection may be necessary. Regular training on the proper use of PPE and emergency procedures should be provided to all personnel involved in handling the chemical.
Exposure limits for 2-Methylpentane must be strictly observed. The Occupational Safety and Health Administration (OSHA) has established a permissible exposure limit (PEL) of 500 parts per million (ppm) for an 8-hour time-weighted average. Monitoring of air quality in the laboratory should be conducted regularly to ensure compliance with these limits.
Proper disposal of 2-Methylpentane and related waste is critical. The chemical should not be released into drains or the environment. Waste should be collected in appropriate containers and disposed of through licensed waste management facilities in accordance with local, state, and federal regulations.
Emergency response procedures must be clearly defined and communicated. This includes protocols for chemical spills, fires, and accidental exposure. Safety showers and eyewash stations should be readily accessible in the laboratory. Material Safety Data Sheets (MSDS) for 2-Methylpentane must be available and easily accessible to all personnel.
Compliance with relevant regulations is mandatory. This includes adherence to the guidelines set forth by OSHA, the Environmental Protection Agency (EPA), and other applicable regulatory bodies. Regular safety audits and inspections should be conducted to ensure ongoing compliance and identify areas for improvement in safety protocols.
Lastly, proper documentation and record-keeping are essential. This includes maintaining logs of chemical usage, exposure monitoring results, and any incidents or near-misses. These records are crucial for regulatory compliance, continuous improvement of safety measures, and potential future research on the long-term effects of 2-Methylpentane exposure.
Proper handling and storage of 2-Methylpentane are paramount. The chemical should be kept in tightly sealed containers in a cool, well-ventilated area away from sources of ignition. Due to its high flammability, fire safety measures must be in place, including readily accessible fire extinguishers and sprinkler systems. Laboratories should be equipped with fume hoods or local exhaust ventilation systems to minimize exposure to vapors.
Personal protective equipment (PPE) is essential when working with 2-Methylpentane. This includes chemical-resistant gloves, safety goggles, and lab coats. In cases where exposure limits may be exceeded, respiratory protection may be necessary. Regular training on the proper use of PPE and emergency procedures should be provided to all personnel involved in handling the chemical.
Exposure limits for 2-Methylpentane must be strictly observed. The Occupational Safety and Health Administration (OSHA) has established a permissible exposure limit (PEL) of 500 parts per million (ppm) for an 8-hour time-weighted average. Monitoring of air quality in the laboratory should be conducted regularly to ensure compliance with these limits.
Proper disposal of 2-Methylpentane and related waste is critical. The chemical should not be released into drains or the environment. Waste should be collected in appropriate containers and disposed of through licensed waste management facilities in accordance with local, state, and federal regulations.
Emergency response procedures must be clearly defined and communicated. This includes protocols for chemical spills, fires, and accidental exposure. Safety showers and eyewash stations should be readily accessible in the laboratory. Material Safety Data Sheets (MSDS) for 2-Methylpentane must be available and easily accessible to all personnel.
Compliance with relevant regulations is mandatory. This includes adherence to the guidelines set forth by OSHA, the Environmental Protection Agency (EPA), and other applicable regulatory bodies. Regular safety audits and inspections should be conducted to ensure ongoing compliance and identify areas for improvement in safety protocols.
Lastly, proper documentation and record-keeping are essential. This includes maintaining logs of chemical usage, exposure monitoring results, and any incidents or near-misses. These records are crucial for regulatory compliance, continuous improvement of safety measures, and potential future research on the long-term effects of 2-Methylpentane exposure.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!