2-Methylpentane Utilization in Solvent Extraction Techniques
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2-Methylpentane Background and Objectives
2-Methylpentane, a branched alkane with the molecular formula C6H14, has emerged as a significant compound in solvent extraction techniques. This isomer of hexane has gained attention due to its unique properties that make it particularly suitable for various extraction processes. The evolution of 2-methylpentane's use in solvent extraction can be traced back to the mid-20th century when the petrochemical industry began exploring more efficient and selective solvents for separation processes.
The primary objective of utilizing 2-methylpentane in solvent extraction techniques is to enhance the efficiency and selectivity of separation processes across various industries. Its low boiling point, high volatility, and excellent solvency for non-polar compounds make it an attractive choice for extracting organic substances from complex mixtures. These properties have led to its widespread adoption in the pharmaceutical, food, and chemical industries.
In the pharmaceutical sector, 2-methylpentane has been instrumental in the extraction of active pharmaceutical ingredients (APIs) from natural sources and in the purification of synthetic compounds. Its ability to selectively dissolve certain organic molecules while leaving others behind has made it invaluable in the isolation of specific compounds from complex biological matrices.
The food industry has also benefited from 2-methylpentane's extraction capabilities, particularly in the production of flavors, fragrances, and essential oils. Its low toxicity compared to other hydrocarbon solvents has made it a preferred choice for applications where residual solvent levels are a concern. The compound's effectiveness in extracting lipophilic components from plant materials has led to its use in the production of high-quality extracts for both culinary and cosmetic applications.
In the realm of analytical chemistry, 2-methylpentane has found applications in chromatographic techniques, serving as a mobile phase component in liquid chromatography and as an extraction solvent in sample preparation methods. Its role in these analytical processes has contributed to the development of more sensitive and accurate analytical methods for complex sample matrices.
The technological trajectory of 2-methylpentane in solvent extraction has been marked by continuous improvements in its production methods, purification techniques, and application-specific formulations. Research efforts have focused on optimizing its use in conjunction with other solvents and developing novel extraction methodologies that leverage its unique properties.
As environmental concerns have grown, there has been an increased emphasis on developing greener extraction processes. This has led to investigations into the potential of 2-methylpentane as a more environmentally friendly alternative to traditional chlorinated solvents in certain applications. The ongoing research aims to balance its extraction efficiency with environmental sustainability, exploring ways to minimize its environmental impact through improved recovery and recycling methods.
The primary objective of utilizing 2-methylpentane in solvent extraction techniques is to enhance the efficiency and selectivity of separation processes across various industries. Its low boiling point, high volatility, and excellent solvency for non-polar compounds make it an attractive choice for extracting organic substances from complex mixtures. These properties have led to its widespread adoption in the pharmaceutical, food, and chemical industries.
In the pharmaceutical sector, 2-methylpentane has been instrumental in the extraction of active pharmaceutical ingredients (APIs) from natural sources and in the purification of synthetic compounds. Its ability to selectively dissolve certain organic molecules while leaving others behind has made it invaluable in the isolation of specific compounds from complex biological matrices.
The food industry has also benefited from 2-methylpentane's extraction capabilities, particularly in the production of flavors, fragrances, and essential oils. Its low toxicity compared to other hydrocarbon solvents has made it a preferred choice for applications where residual solvent levels are a concern. The compound's effectiveness in extracting lipophilic components from plant materials has led to its use in the production of high-quality extracts for both culinary and cosmetic applications.
In the realm of analytical chemistry, 2-methylpentane has found applications in chromatographic techniques, serving as a mobile phase component in liquid chromatography and as an extraction solvent in sample preparation methods. Its role in these analytical processes has contributed to the development of more sensitive and accurate analytical methods for complex sample matrices.
The technological trajectory of 2-methylpentane in solvent extraction has been marked by continuous improvements in its production methods, purification techniques, and application-specific formulations. Research efforts have focused on optimizing its use in conjunction with other solvents and developing novel extraction methodologies that leverage its unique properties.
As environmental concerns have grown, there has been an increased emphasis on developing greener extraction processes. This has led to investigations into the potential of 2-methylpentane as a more environmentally friendly alternative to traditional chlorinated solvents in certain applications. The ongoing research aims to balance its extraction efficiency with environmental sustainability, exploring ways to minimize its environmental impact through improved recovery and recycling methods.
Solvent Extraction Market Analysis
The solvent extraction market has been experiencing steady growth, driven by increasing demand across various industries such as pharmaceuticals, petrochemicals, and food processing. The global market for solvent extraction is projected to expand significantly in the coming years, with a compound annual growth rate (CAGR) exceeding the average industrial growth rate. This growth is primarily attributed to the rising need for efficient separation and purification processes in manufacturing and research applications.
In the context of 2-Methylpentane utilization in solvent extraction techniques, the market analysis reveals a niche but growing segment within the broader solvent extraction landscape. 2-Methylpentane, an isomer of hexane, is gaining attention due to its favorable properties as a solvent, including low boiling point, high selectivity, and relatively low toxicity compared to some traditional solvents.
The pharmaceutical industry represents a key market for 2-Methylpentane-based solvent extraction, particularly in the production of active pharmaceutical ingredients (APIs) and natural product extraction. The increasing focus on green chemistry and sustainable manufacturing processes has led to a growing interest in alternative solvents like 2-Methylpentane, which offers improved environmental profiles compared to some conventional options.
In the petrochemical sector, 2-Methylpentane is finding applications in the extraction of valuable components from complex hydrocarbon mixtures. Its use in this industry is expected to grow as refineries and chemical plants seek more efficient and environmentally friendly separation processes.
The food and beverage industry is another significant market for 2-Methylpentane-based solvent extraction, particularly in the extraction of flavors, fragrances, and essential oils. The demand for natural and clean-label products is driving the adoption of safer and more effective extraction solvents, positioning 2-Methylpentane as a promising alternative to traditional solvents.
Geographically, North America and Europe are currently the leading markets for advanced solvent extraction techniques, including those utilizing 2-Methylpentane. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing research and development activities, and growing awareness of sustainable manufacturing practices.
The market for 2-Methylpentane in solvent extraction is characterized by a relatively small number of suppliers, with potential for new entrants as demand grows. Key challenges in market expansion include regulatory hurdles, particularly in food and pharmaceutical applications, and the need for extensive validation of new extraction processes in regulated industries.
In the context of 2-Methylpentane utilization in solvent extraction techniques, the market analysis reveals a niche but growing segment within the broader solvent extraction landscape. 2-Methylpentane, an isomer of hexane, is gaining attention due to its favorable properties as a solvent, including low boiling point, high selectivity, and relatively low toxicity compared to some traditional solvents.
The pharmaceutical industry represents a key market for 2-Methylpentane-based solvent extraction, particularly in the production of active pharmaceutical ingredients (APIs) and natural product extraction. The increasing focus on green chemistry and sustainable manufacturing processes has led to a growing interest in alternative solvents like 2-Methylpentane, which offers improved environmental profiles compared to some conventional options.
In the petrochemical sector, 2-Methylpentane is finding applications in the extraction of valuable components from complex hydrocarbon mixtures. Its use in this industry is expected to grow as refineries and chemical plants seek more efficient and environmentally friendly separation processes.
The food and beverage industry is another significant market for 2-Methylpentane-based solvent extraction, particularly in the extraction of flavors, fragrances, and essential oils. The demand for natural and clean-label products is driving the adoption of safer and more effective extraction solvents, positioning 2-Methylpentane as a promising alternative to traditional solvents.
Geographically, North America and Europe are currently the leading markets for advanced solvent extraction techniques, including those utilizing 2-Methylpentane. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing research and development activities, and growing awareness of sustainable manufacturing practices.
The market for 2-Methylpentane in solvent extraction is characterized by a relatively small number of suppliers, with potential for new entrants as demand grows. Key challenges in market expansion include regulatory hurdles, particularly in food and pharmaceutical applications, and the need for extensive validation of new extraction processes in regulated industries.
2-Methylpentane Extraction Challenges
The utilization of 2-methylpentane in solvent extraction techniques presents several significant challenges that researchers and industry professionals must address. One of the primary obstacles is the compound's relatively high volatility, which can lead to substantial solvent losses during the extraction process. This volatility not only impacts the efficiency of the extraction but also raises environmental concerns due to potential emissions of volatile organic compounds (VOCs).
Another challenge lies in the selectivity of 2-methylpentane as an extraction solvent. While it demonstrates good solvency for certain non-polar compounds, its effectiveness can be limited when dealing with more polar analytes. This selectivity issue necessitates careful consideration of the target compounds and may require the development of more complex solvent systems or extraction protocols to achieve optimal results.
The potential for emulsion formation during liquid-liquid extraction processes involving 2-methylpentane poses yet another hurdle. Emulsions can significantly impede phase separation, leading to reduced extraction efficiency and increased processing times. Overcoming this challenge often requires the implementation of additional separation techniques or the use of demulsifying agents, which can add complexity and cost to the extraction process.
Safety considerations also play a crucial role in the challenges associated with 2-methylpentane extraction. The compound's high flammability and potential for forming explosive mixtures with air necessitate stringent safety protocols and specialized equipment. This not only increases operational costs but also requires extensive training for personnel involved in handling the solvent.
From an analytical perspective, the interference of 2-methylpentane with certain detection methods can complicate the quantification of extracted analytes. This is particularly problematic in trace analysis, where the solvent signal may overshadow the analytes of interest. Developing robust analytical methods that can effectively separate and quantify target compounds in the presence of 2-methylpentane remains an ongoing challenge.
The environmental impact of 2-methylpentane usage in extraction processes is another area of concern. As a volatile organic compound, it contributes to air pollution and potentially to the formation of ground-level ozone. Regulatory pressures to reduce VOC emissions are driving the need for more environmentally friendly alternatives or improved containment and recovery systems for 2-methylpentane.
Lastly, the economic feasibility of using 2-methylpentane in large-scale extraction processes presents a challenge. The cost of the solvent, coupled with the expenses associated with its safe handling and potential recovery systems, can impact the overall economics of extraction operations. Balancing these costs against the benefits of using 2-methylpentane is a critical consideration for industrial applications.
Another challenge lies in the selectivity of 2-methylpentane as an extraction solvent. While it demonstrates good solvency for certain non-polar compounds, its effectiveness can be limited when dealing with more polar analytes. This selectivity issue necessitates careful consideration of the target compounds and may require the development of more complex solvent systems or extraction protocols to achieve optimal results.
The potential for emulsion formation during liquid-liquid extraction processes involving 2-methylpentane poses yet another hurdle. Emulsions can significantly impede phase separation, leading to reduced extraction efficiency and increased processing times. Overcoming this challenge often requires the implementation of additional separation techniques or the use of demulsifying agents, which can add complexity and cost to the extraction process.
Safety considerations also play a crucial role in the challenges associated with 2-methylpentane extraction. The compound's high flammability and potential for forming explosive mixtures with air necessitate stringent safety protocols and specialized equipment. This not only increases operational costs but also requires extensive training for personnel involved in handling the solvent.
From an analytical perspective, the interference of 2-methylpentane with certain detection methods can complicate the quantification of extracted analytes. This is particularly problematic in trace analysis, where the solvent signal may overshadow the analytes of interest. Developing robust analytical methods that can effectively separate and quantify target compounds in the presence of 2-methylpentane remains an ongoing challenge.
The environmental impact of 2-methylpentane usage in extraction processes is another area of concern. As a volatile organic compound, it contributes to air pollution and potentially to the formation of ground-level ozone. Regulatory pressures to reduce VOC emissions are driving the need for more environmentally friendly alternatives or improved containment and recovery systems for 2-methylpentane.
Lastly, the economic feasibility of using 2-methylpentane in large-scale extraction processes presents a challenge. The cost of the solvent, coupled with the expenses associated with its safe handling and potential recovery systems, can impact the overall economics of extraction operations. Balancing these costs against the benefits of using 2-methylpentane is a critical consideration for industrial applications.
Current 2-Methylpentane Extraction Methods
01 Use as a solvent in chemical processes
2-Methylpentane is utilized as a solvent in various chemical processes, particularly in the production of polymers and other organic compounds. Its properties make it suitable for dissolving and processing certain materials, enhancing reaction efficiency and product quality.- Use as a solvent in chemical processes: 2-Methylpentane is utilized as a solvent in various chemical processes, particularly in the production of polymers and other organic compounds. Its properties make it suitable for dissolving and processing certain materials in industrial applications.
- Component in fuel formulations: 2-Methylpentane is used as a component in fuel formulations, particularly in gasoline blends. It contributes to the octane rating and overall performance of the fuel, enhancing combustion efficiency in internal combustion engines.
- Application in catalytic processes: 2-Methylpentane is involved in various catalytic processes, including isomerization and reforming reactions. It serves as a reactant or intermediate in the production of other hydrocarbons and petrochemicals.
- Use in separation and purification processes: 2-Methylpentane is employed in separation and purification processes, particularly in the petrochemical industry. It can be used as an extractant or in distillation processes to separate and purify other hydrocarbons.
- Environmental and safety considerations: The use of 2-Methylpentane in various applications requires consideration of environmental and safety factors. This includes its potential impact on air quality, handling precautions, and regulatory compliance in industrial settings.
02 Component in fuel compositions
2-Methylpentane is employed as a component in fuel compositions, particularly for internal combustion engines. It can be used to improve the octane rating and overall performance of gasoline blends, contributing to better engine efficiency and reduced emissions.Expand Specific Solutions03 Application in separation processes
2-Methylpentane is used in separation processes, such as extractive distillation or liquid-liquid extraction. Its unique physical properties allow for the effective separation of mixtures, particularly in the purification of hydrocarbon streams or the isolation of specific compounds.Expand Specific Solutions04 Precursor in chemical synthesis
2-Methylpentane serves as a precursor or starting material in the synthesis of various organic compounds. It can undergo reactions such as isomerization, dehydrogenation, or functionalization to produce more complex molecules with applications in pharmaceuticals, agrochemicals, or specialty chemicals.Expand Specific Solutions05 Use in analytical chemistry
2-Methylpentane finds applications in analytical chemistry, particularly as a reference standard or calibration compound in gas chromatography and other analytical techniques. Its well-defined properties make it useful for instrument calibration and method development in chemical analysis.Expand Specific Solutions
Key Players in Solvent Industry
The utilization of 2-Methylpentane in solvent extraction techniques is an emerging field within the petrochemical industry. The market is in its early growth stage, with increasing demand for efficient and environmentally friendly solvents driving research and development. While the global market size is still relatively small, it is expected to grow significantly in the coming years. Technologically, the process is advancing rapidly, with companies like China Petroleum & Chemical Corp., Sinopec Shanghai Petrochemical Co., and Shandong Chambroad Petrochemicals leading the way in developing innovative applications. These firms are investing heavily in R&D to improve extraction efficiency and reduce environmental impact, indicating a moderate level of technological maturity with room for further advancements.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced solvent extraction technique utilizing 2-methylpentane for enhanced oil recovery and petrochemical processing. Their method involves a multi-stage extraction process where 2-methylpentane is used as a selective solvent to separate aromatic compounds from aliphatic hydrocarbons in complex mixtures[1]. The process employs a counter-current extraction column operating at optimized temperatures and pressures to maximize separation efficiency. Sinopec has also implemented a solvent recovery system that recycles up to 98% of the 2-methylpentane, significantly reducing operational costs and environmental impact[2]. Additionally, they have developed a proprietary catalyst that enhances the selectivity of 2-methylpentane in the extraction process, improving overall yield and purity of target compounds[3].
Strengths: High separation efficiency, excellent solvent recovery rate, and improved selectivity through catalyst use. Weaknesses: Potential safety concerns due to the flammability of 2-methylpentane, and the need for specialized equipment for high-pressure operations.
SINOPEC Beijing Research Institute of Chemical Industry
Technical Solution: SINOPEC Beijing Research Institute of Chemical Industry has pioneered a novel approach to 2-methylpentane utilization in solvent extraction techniques, focusing on the pharmaceutical and fine chemical industries. Their method incorporates a continuous-flow microreactor system that allows for precise control of reaction conditions and improved mass transfer[4]. This system enables the use of 2-methylpentane as both a reaction medium and extraction solvent, streamlining the production process for various high-value compounds. The institute has also developed a green chemistry approach, using supercritical 2-methylpentane for extraction, which operates at lower temperatures and reduces energy consumption by up to 40% compared to conventional methods[5]. Furthermore, they have implemented an in-line analytical system that provides real-time monitoring of the extraction process, allowing for rapid optimization and quality control[6].
Strengths: Innovative continuous-flow system, energy-efficient supercritical extraction, and real-time process monitoring. Weaknesses: Higher initial investment costs for specialized equipment and potential scalability challenges for larger production volumes.
Core Innovations in 2-Methylpentane Extraction
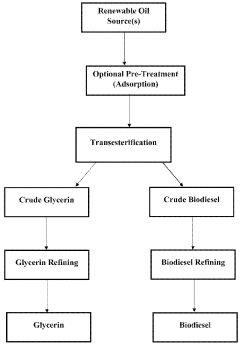
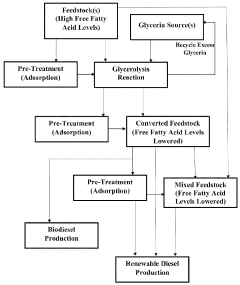
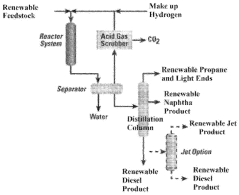
Renewable fuels, diesel and methods of generation from renewable oil sources
PatentWO2023147174A1
Innovation
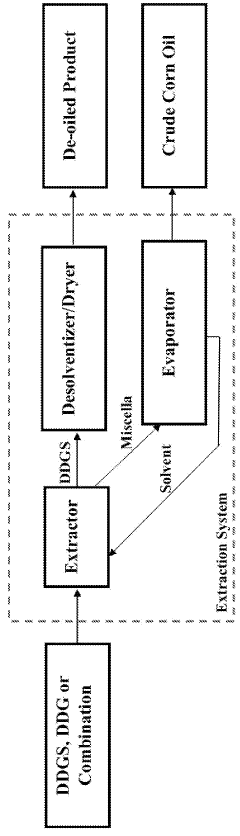
- A process involving glycerolysis of renewable oil feedstocks, particularly crude corn oil from DDGS and DDG, in the presence of a molar excess of glycerin to reduce FFA content, converting triglycerides to monoglycerides and diglycerides, thereby creating a feedstock suitable for renewable fuel production.
Method for extracting 2-methylbutane aromatic compounds
PatentWO2022122458A1
Innovation
- A process utilizing a polar solvent and an auxiliary solvent, 2-methylbutane, in a countercurrent extraction method that reduces energy consumption and thermal level, allowing for efficient separation of aromatic and non-aromatic compounds, even in extended hydrocarbon cuts, by using 2-methylbutane as an auxiliary solvent to facilitate the separation of heavy aromatic compounds.
Environmental Impact Assessment
The utilization of 2-methylpentane in solvent extraction techniques raises significant environmental concerns that require thorough assessment. This hydrocarbon, a component of petroleum and natural gas, poses potential risks to ecosystems and human health if not properly managed. When used in solvent extraction processes, 2-methylpentane can volatilize and contribute to air pollution, particularly in the form of volatile organic compounds (VOCs). These emissions may lead to the formation of ground-level ozone and smog, negatively impacting air quality and respiratory health in surrounding communities.
Water contamination is another critical environmental issue associated with 2-methylpentane usage. Accidental spills or improper disposal of extraction waste can result in the compound leaching into groundwater or surface water bodies. This contamination can have far-reaching effects on aquatic ecosystems, potentially disrupting food chains and biodiversity. Moreover, the persistence of 2-methylpentane in water systems may lead to long-term environmental degradation and pose challenges for water treatment facilities.
Soil contamination is also a concern, particularly in areas where solvent extraction processes are conducted. The compound's ability to adsorb to soil particles can lead to the accumulation of pollutants in the soil matrix, potentially affecting soil fertility and microbial communities. This contamination may have cascading effects on terrestrial ecosystems and agricultural productivity in affected areas.
The environmental impact of 2-methylpentane extends to its carbon footprint and contribution to climate change. As a petroleum-derived compound, its production and use are associated with greenhouse gas emissions throughout its lifecycle. The energy-intensive nature of solvent extraction processes further exacerbates this environmental burden, necessitating a comprehensive evaluation of the technique's carbon intensity and potential alternatives.
Biodegradation and bioaccumulation are additional factors to consider in the environmental impact assessment. While 2-methylpentane can undergo biodegradation under certain conditions, its rate of breakdown in the environment may vary depending on factors such as temperature, microbial activity, and the presence of other pollutants. The potential for bioaccumulation in aquatic organisms and subsequent biomagnification through food chains warrants careful monitoring and risk assessment.
To mitigate these environmental impacts, it is crucial to implement robust containment and treatment systems in facilities utilizing 2-methylpentane for solvent extraction. This includes the use of advanced air pollution control technologies, wastewater treatment systems, and proper waste management protocols. Additionally, exploring greener alternatives or optimizing extraction processes to reduce solvent usage can significantly minimize the environmental footprint of these operations.
Water contamination is another critical environmental issue associated with 2-methylpentane usage. Accidental spills or improper disposal of extraction waste can result in the compound leaching into groundwater or surface water bodies. This contamination can have far-reaching effects on aquatic ecosystems, potentially disrupting food chains and biodiversity. Moreover, the persistence of 2-methylpentane in water systems may lead to long-term environmental degradation and pose challenges for water treatment facilities.
Soil contamination is also a concern, particularly in areas where solvent extraction processes are conducted. The compound's ability to adsorb to soil particles can lead to the accumulation of pollutants in the soil matrix, potentially affecting soil fertility and microbial communities. This contamination may have cascading effects on terrestrial ecosystems and agricultural productivity in affected areas.
The environmental impact of 2-methylpentane extends to its carbon footprint and contribution to climate change. As a petroleum-derived compound, its production and use are associated with greenhouse gas emissions throughout its lifecycle. The energy-intensive nature of solvent extraction processes further exacerbates this environmental burden, necessitating a comprehensive evaluation of the technique's carbon intensity and potential alternatives.
Biodegradation and bioaccumulation are additional factors to consider in the environmental impact assessment. While 2-methylpentane can undergo biodegradation under certain conditions, its rate of breakdown in the environment may vary depending on factors such as temperature, microbial activity, and the presence of other pollutants. The potential for bioaccumulation in aquatic organisms and subsequent biomagnification through food chains warrants careful monitoring and risk assessment.
To mitigate these environmental impacts, it is crucial to implement robust containment and treatment systems in facilities utilizing 2-methylpentane for solvent extraction. This includes the use of advanced air pollution control technologies, wastewater treatment systems, and proper waste management protocols. Additionally, exploring greener alternatives or optimizing extraction processes to reduce solvent usage can significantly minimize the environmental footprint of these operations.
Safety Regulations for 2-Methylpentane Use
The utilization of 2-methylpentane in solvent extraction techniques necessitates strict adherence to safety regulations to mitigate potential risks associated with its use. These regulations encompass various aspects of handling, storage, and disposal of this volatile organic compound.
Occupational safety standards require proper personal protective equipment (PPE) when working with 2-methylpentane. This includes chemical-resistant gloves, safety goggles, and appropriate respiratory protection. Workplace ventilation systems must meet specific requirements to prevent the accumulation of vapors, which can pose fire and health hazards.
Storage regulations mandate that 2-methylpentane be kept in tightly sealed containers in well-ventilated areas, away from sources of ignition and incompatible materials. Temperature control is crucial, as the compound's low flash point increases fire risk. Facilities must implement proper grounding and bonding procedures to prevent static electricity buildup during transfer operations.
Emergency response protocols are essential components of safety regulations. Facilities using 2-methylpentane must have readily accessible fire suppression systems and spill containment equipment. Personnel should be trained in proper emergency procedures, including evacuation plans and the use of fire extinguishers suitable for flammable liquid fires.
Environmental protection regulations govern the disposal of 2-methylpentane and its waste products. The compound is classified as hazardous waste in many jurisdictions, requiring specialized handling and disposal methods to prevent environmental contamination. Facilities must maintain detailed records of waste generation, storage, and disposal in compliance with local and national regulations.
Transportation of 2-methylpentane is subject to stringent regulations due to its flammability. It is classified as a hazardous material for shipping purposes, requiring proper labeling, packaging, and documentation. Carriers must comply with specific routing and quantity restrictions, and drivers transporting the compound need specialized training and certifications.
Workplace exposure limits for 2-methylpentane are established by regulatory bodies such as OSHA in the United States. These limits define the maximum allowable concentration of the compound in workplace air over specified time periods. Regular air quality monitoring and employee health surveillance programs are often mandated to ensure compliance with these exposure limits.
Safety data sheets (SDS) play a crucial role in communicating hazard information and safe handling practices for 2-methylpentane. Regulations require that up-to-date SDS be readily available to all personnel working with or potentially exposed to the compound. These documents must provide comprehensive information on physical and chemical properties, health hazards, first aid measures, and proper handling procedures.
Occupational safety standards require proper personal protective equipment (PPE) when working with 2-methylpentane. This includes chemical-resistant gloves, safety goggles, and appropriate respiratory protection. Workplace ventilation systems must meet specific requirements to prevent the accumulation of vapors, which can pose fire and health hazards.
Storage regulations mandate that 2-methylpentane be kept in tightly sealed containers in well-ventilated areas, away from sources of ignition and incompatible materials. Temperature control is crucial, as the compound's low flash point increases fire risk. Facilities must implement proper grounding and bonding procedures to prevent static electricity buildup during transfer operations.
Emergency response protocols are essential components of safety regulations. Facilities using 2-methylpentane must have readily accessible fire suppression systems and spill containment equipment. Personnel should be trained in proper emergency procedures, including evacuation plans and the use of fire extinguishers suitable for flammable liquid fires.
Environmental protection regulations govern the disposal of 2-methylpentane and its waste products. The compound is classified as hazardous waste in many jurisdictions, requiring specialized handling and disposal methods to prevent environmental contamination. Facilities must maintain detailed records of waste generation, storage, and disposal in compliance with local and national regulations.
Transportation of 2-methylpentane is subject to stringent regulations due to its flammability. It is classified as a hazardous material for shipping purposes, requiring proper labeling, packaging, and documentation. Carriers must comply with specific routing and quantity restrictions, and drivers transporting the compound need specialized training and certifications.
Workplace exposure limits for 2-methylpentane are established by regulatory bodies such as OSHA in the United States. These limits define the maximum allowable concentration of the compound in workplace air over specified time periods. Regular air quality monitoring and employee health surveillance programs are often mandated to ensure compliance with these exposure limits.
Safety data sheets (SDS) play a crucial role in communicating hazard information and safe handling practices for 2-methylpentane. Regulations require that up-to-date SDS be readily available to all personnel working with or potentially exposed to the compound. These documents must provide comprehensive information on physical and chemical properties, health hazards, first aid measures, and proper handling procedures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!