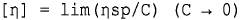Stability Analysis of 2-Methylpentane in Extreme Temperature Conditions
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Background and Objectives
The stability analysis of 2-methylpentane under extreme temperature conditions has become increasingly important in various industrial applications, particularly in the petrochemical and energy sectors. This branched alkane, also known as isohexane, plays a crucial role in fuel blending, solvent production, and as a precursor in chemical synthesis. Understanding its behavior and stability across a wide range of temperatures is essential for optimizing processes, ensuring safety, and developing new applications.
The primary objective of this technical research is to comprehensively examine the stability characteristics of 2-methylpentane when subjected to extreme temperature conditions, both at the upper and lower ends of the spectrum. This investigation aims to provide valuable insights into the compound's physical and chemical properties, phase transitions, and potential structural changes that may occur under these challenging environments.
Historically, the study of hydrocarbon stability has been driven by the needs of the oil and gas industry, aerospace applications, and the automotive sector. As these industries continue to push the boundaries of operational conditions, the demand for detailed stability analyses of key compounds like 2-methylpentane has grown significantly. Recent advancements in analytical techniques and computational modeling have opened new avenues for more precise and in-depth investigations of molecular behavior under extreme conditions.
The evolution of this field has seen a shift from purely empirical approaches to more sophisticated methods combining experimental data with theoretical predictions. This progression has enabled researchers to gain deeper insights into the fundamental mechanisms governing the stability of hydrocarbons at molecular levels. For 2-methylpentane, understanding these mechanisms is particularly important due to its unique branched structure, which can influence its reactivity and stability compared to its straight-chain isomers.
In the context of current technological trends, this research aligns with the broader goals of developing more efficient and environmentally friendly energy solutions. As the world transitions towards cleaner energy sources, understanding the behavior of hydrocarbons like 2-methylpentane under various conditions becomes crucial for optimizing existing processes and exploring new applications in emerging fields such as advanced materials and nanotechnology.
This technical research report aims to consolidate existing knowledge, present new findings, and identify key areas for future investigation regarding the stability of 2-methylpentane in extreme temperature conditions. By doing so, it seeks to contribute to the broader scientific understanding of hydrocarbon behavior and support innovation in related industrial applications. The outcomes of this study are expected to have significant implications for process design, safety protocols, and product development across multiple sectors relying on the unique properties of 2-methylpentane.
The primary objective of this technical research is to comprehensively examine the stability characteristics of 2-methylpentane when subjected to extreme temperature conditions, both at the upper and lower ends of the spectrum. This investigation aims to provide valuable insights into the compound's physical and chemical properties, phase transitions, and potential structural changes that may occur under these challenging environments.
Historically, the study of hydrocarbon stability has been driven by the needs of the oil and gas industry, aerospace applications, and the automotive sector. As these industries continue to push the boundaries of operational conditions, the demand for detailed stability analyses of key compounds like 2-methylpentane has grown significantly. Recent advancements in analytical techniques and computational modeling have opened new avenues for more precise and in-depth investigations of molecular behavior under extreme conditions.
The evolution of this field has seen a shift from purely empirical approaches to more sophisticated methods combining experimental data with theoretical predictions. This progression has enabled researchers to gain deeper insights into the fundamental mechanisms governing the stability of hydrocarbons at molecular levels. For 2-methylpentane, understanding these mechanisms is particularly important due to its unique branched structure, which can influence its reactivity and stability compared to its straight-chain isomers.
In the context of current technological trends, this research aligns with the broader goals of developing more efficient and environmentally friendly energy solutions. As the world transitions towards cleaner energy sources, understanding the behavior of hydrocarbons like 2-methylpentane under various conditions becomes crucial for optimizing existing processes and exploring new applications in emerging fields such as advanced materials and nanotechnology.
This technical research report aims to consolidate existing knowledge, present new findings, and identify key areas for future investigation regarding the stability of 2-methylpentane in extreme temperature conditions. By doing so, it seeks to contribute to the broader scientific understanding of hydrocarbon behavior and support innovation in related industrial applications. The outcomes of this study are expected to have significant implications for process design, safety protocols, and product development across multiple sectors relying on the unique properties of 2-methylpentane.
Market Demand Analysis
The market demand for stability analysis of 2-methylpentane in extreme temperature conditions is driven by several key factors in the petrochemical and industrial sectors. As a branched isomer of hexane, 2-methylpentane finds extensive use as a solvent, fuel component, and chemical intermediate. Its stability under extreme temperatures is crucial for various applications, particularly in the automotive, aerospace, and manufacturing industries.
In the automotive sector, the demand for high-performance fuels capable of withstanding extreme temperature fluctuations continues to grow. 2-methylpentane, as a component in gasoline blends, requires thorough stability analysis to ensure optimal engine performance and fuel efficiency across diverse climatic conditions. This need is further amplified by the increasing adoption of turbocharged engines and the push for higher octane fuels, where the stability of fuel components becomes paramount.
The aerospace industry presents another significant market for 2-methylpentane stability analysis. With aircraft operating in environments ranging from scorching deserts to sub-zero stratospheric conditions, the stability of fuel components and hydraulic fluids is critical for safety and performance. The demand for reliable stability data on 2-methylpentane under these extreme conditions is expected to rise as the aerospace sector continues to expand and innovate.
In the manufacturing sector, 2-methylpentane's use as a solvent in various processes necessitates a comprehensive understanding of its stability across a wide temperature range. Industries such as electronics, pharmaceuticals, and specialty chemicals rely on solvents that maintain their properties under diverse processing conditions. The growing emphasis on quality control and process optimization in these sectors is likely to drive increased demand for detailed stability analyses of 2-methylpentane.
The global push towards sustainability and environmental responsibility also influences the market demand for 2-methylpentane stability analysis. As industries seek to reduce emissions and improve energy efficiency, understanding the behavior of chemical components under extreme conditions becomes crucial for developing more environmentally friendly products and processes. This trend is expected to fuel research and development efforts focused on the stability characteristics of 2-methylpentane and similar compounds.
Furthermore, regulatory bodies worldwide are imposing stricter guidelines on the use and handling of chemical substances, including 2-methylpentane. This regulatory landscape creates a demand for comprehensive stability data to ensure compliance with safety standards and environmental regulations. Companies across various industries are increasingly investing in stability analyses to mitigate risks associated with the use of 2-methylpentane in extreme temperature conditions.
In the automotive sector, the demand for high-performance fuels capable of withstanding extreme temperature fluctuations continues to grow. 2-methylpentane, as a component in gasoline blends, requires thorough stability analysis to ensure optimal engine performance and fuel efficiency across diverse climatic conditions. This need is further amplified by the increasing adoption of turbocharged engines and the push for higher octane fuels, where the stability of fuel components becomes paramount.
The aerospace industry presents another significant market for 2-methylpentane stability analysis. With aircraft operating in environments ranging from scorching deserts to sub-zero stratospheric conditions, the stability of fuel components and hydraulic fluids is critical for safety and performance. The demand for reliable stability data on 2-methylpentane under these extreme conditions is expected to rise as the aerospace sector continues to expand and innovate.
In the manufacturing sector, 2-methylpentane's use as a solvent in various processes necessitates a comprehensive understanding of its stability across a wide temperature range. Industries such as electronics, pharmaceuticals, and specialty chemicals rely on solvents that maintain their properties under diverse processing conditions. The growing emphasis on quality control and process optimization in these sectors is likely to drive increased demand for detailed stability analyses of 2-methylpentane.
The global push towards sustainability and environmental responsibility also influences the market demand for 2-methylpentane stability analysis. As industries seek to reduce emissions and improve energy efficiency, understanding the behavior of chemical components under extreme conditions becomes crucial for developing more environmentally friendly products and processes. This trend is expected to fuel research and development efforts focused on the stability characteristics of 2-methylpentane and similar compounds.
Furthermore, regulatory bodies worldwide are imposing stricter guidelines on the use and handling of chemical substances, including 2-methylpentane. This regulatory landscape creates a demand for comprehensive stability data to ensure compliance with safety standards and environmental regulations. Companies across various industries are increasingly investing in stability analyses to mitigate risks associated with the use of 2-methylpentane in extreme temperature conditions.
Current Challenges
The stability analysis of 2-methylpentane in extreme temperature conditions presents several significant challenges that researchers and industry professionals must address. One of the primary obstacles is the lack of comprehensive experimental data across a wide range of extreme temperatures. While some studies have been conducted, there is a notable gap in understanding the compound's behavior at both extremely low and high temperatures, particularly beyond the typical operational range of industrial processes.
The molecular structure of 2-methylpentane, with its branched carbon chain, introduces complexities in predicting its stability under extreme conditions. The presence of the methyl group attached to the second carbon atom affects the overall molecular geometry and intermolecular forces, making it challenging to accurately model its behavior using conventional simulation techniques. This structural uniqueness necessitates the development of more sophisticated computational models that can account for these specific molecular interactions at varying temperature extremes.
Another significant challenge lies in the potential for phase transitions and structural changes that may occur at extreme temperatures. As the temperature approaches either very low or very high values, 2-methylpentane may undergo unexpected transformations that alter its physical and chemical properties. These changes can include shifts in crystal structure at low temperatures or the onset of thermal decomposition at high temperatures. Accurately predicting and characterizing these phase transitions requires advanced experimental techniques and theoretical frameworks that are not yet fully developed for this specific compound.
The stability of 2-methylpentane is also influenced by its interactions with other substances present in real-world applications. In many industrial processes, 2-methylpentane is not used in isolation but as part of complex mixtures or in the presence of catalysts. Understanding how these interactions affect the compound's stability under extreme temperature conditions adds another layer of complexity to the analysis. Researchers must develop methods to isolate and study these interaction effects while maintaining the extreme temperature environment, which presents significant technical challenges.
Furthermore, the kinetics of potential degradation reactions at extreme temperatures pose a considerable challenge. At very high temperatures, the risk of thermal cracking or other decomposition pathways increases, while at very low temperatures, the formation of unusual molecular clusters or the slowing of certain chemical processes may occur. Developing accurate kinetic models that can predict the rates and mechanisms of these reactions across a broad temperature range is crucial for understanding the long-term stability of 2-methylpentane in various applications.
Lastly, the practical implementation of stability analysis techniques for 2-methylpentane in industrial settings presents its own set of challenges. Designing experimental setups that can maintain extreme temperature conditions while allowing for precise measurements and sample handling requires specialized equipment and expertise. Additionally, ensuring the safety of personnel and equipment when working with potentially volatile organic compounds at extreme temperatures necessitates rigorous safety protocols and engineering controls, further complicating the research and analysis process.
The molecular structure of 2-methylpentane, with its branched carbon chain, introduces complexities in predicting its stability under extreme conditions. The presence of the methyl group attached to the second carbon atom affects the overall molecular geometry and intermolecular forces, making it challenging to accurately model its behavior using conventional simulation techniques. This structural uniqueness necessitates the development of more sophisticated computational models that can account for these specific molecular interactions at varying temperature extremes.
Another significant challenge lies in the potential for phase transitions and structural changes that may occur at extreme temperatures. As the temperature approaches either very low or very high values, 2-methylpentane may undergo unexpected transformations that alter its physical and chemical properties. These changes can include shifts in crystal structure at low temperatures or the onset of thermal decomposition at high temperatures. Accurately predicting and characterizing these phase transitions requires advanced experimental techniques and theoretical frameworks that are not yet fully developed for this specific compound.
The stability of 2-methylpentane is also influenced by its interactions with other substances present in real-world applications. In many industrial processes, 2-methylpentane is not used in isolation but as part of complex mixtures or in the presence of catalysts. Understanding how these interactions affect the compound's stability under extreme temperature conditions adds another layer of complexity to the analysis. Researchers must develop methods to isolate and study these interaction effects while maintaining the extreme temperature environment, which presents significant technical challenges.
Furthermore, the kinetics of potential degradation reactions at extreme temperatures pose a considerable challenge. At very high temperatures, the risk of thermal cracking or other decomposition pathways increases, while at very low temperatures, the formation of unusual molecular clusters or the slowing of certain chemical processes may occur. Developing accurate kinetic models that can predict the rates and mechanisms of these reactions across a broad temperature range is crucial for understanding the long-term stability of 2-methylpentane in various applications.
Lastly, the practical implementation of stability analysis techniques for 2-methylpentane in industrial settings presents its own set of challenges. Designing experimental setups that can maintain extreme temperature conditions while allowing for precise measurements and sample handling requires specialized equipment and expertise. Additionally, ensuring the safety of personnel and equipment when working with potentially volatile organic compounds at extreme temperatures necessitates rigorous safety protocols and engineering controls, further complicating the research and analysis process.
Existing Solutions
01 Chemical stability of 2-methylpentane
2-Methylpentane exhibits good chemical stability due to its saturated hydrocarbon structure. It is resistant to many chemical reactions under normal conditions, making it suitable for various industrial applications. The stability of 2-methylpentane is influenced by factors such as temperature, pressure, and the presence of catalysts or other reactive substances.- Chemical stability of 2-methylpentane: 2-Methylpentane exhibits good chemical stability due to its saturated hydrocarbon structure. It is resistant to many chemical reactions under normal conditions, making it suitable for various industrial applications where stability is crucial.
- Thermal stability of 2-methylpentane: 2-Methylpentane demonstrates good thermal stability within a certain temperature range. This property makes it useful in applications where heat resistance is required, such as in certain industrial processes or as a component in heat transfer fluids.
- Oxidation stability of 2-methylpentane: 2-Methylpentane shows resistance to oxidation under normal conditions. This stability against oxidation makes it suitable for use in various applications where exposure to air or oxygen is expected, such as in certain lubricants or solvents.
- Storage and handling stability of 2-methylpentane: 2-Methylpentane can be stored and handled safely under appropriate conditions. It maintains its properties during storage and does not degrade significantly over time when properly contained, making it suitable for various industrial and commercial applications.
- Environmental stability of 2-methylpentane: 2-Methylpentane has moderate environmental stability. While it can persist in certain environmental conditions, it may undergo degradation processes in others. Understanding its environmental behavior is important for assessing its potential impact and determining appropriate handling and disposal methods.
02 Thermal stability of 2-methylpentane
2-Methylpentane demonstrates good thermal stability within a certain temperature range. It maintains its chemical structure and properties under moderate heat conditions. However, at very high temperatures, it may undergo thermal decomposition or isomerization. The thermal stability of 2-methylpentane is an important consideration in applications involving heat exposure or thermal processes.Expand Specific Solutions03 Oxidative stability of 2-methylpentane
2-Methylpentane shows resistance to oxidation under normal atmospheric conditions. However, in the presence of strong oxidizing agents or at elevated temperatures, it may undergo oxidation reactions. The oxidative stability of 2-methylpentane is crucial in applications where exposure to oxygen or other oxidizing environments is expected.Expand Specific Solutions04 Storage and handling stability of 2-methylpentane
2-Methylpentane is generally stable during storage and handling under appropriate conditions. It should be kept in sealed containers away from heat sources, ignition sources, and incompatible materials. Proper storage and handling practices are essential to maintain the stability and purity of 2-methylpentane over extended periods.Expand Specific Solutions05 Stability enhancement methods for 2-methylpentane
Various methods can be employed to enhance the stability of 2-methylpentane in specific applications. These may include the use of stabilizers, antioxidants, or other additives to improve its resistance to degradation. Additionally, controlling environmental factors such as temperature, pressure, and exposure to reactive substances can help maintain the stability of 2-methylpentane in industrial processes.Expand Specific Solutions
Key Industry Players
The stability analysis of 2-Methylpentane in extreme temperature conditions represents a niche but critical area within the petrochemical industry. The market is in a mature stage, with established players like ExxonMobil Chemical Patents, Inc. and Mitsui Chemicals, Inc. leading research efforts. The global market size for this specific analysis is relatively small but integral to broader petrochemical applications. Technologically, the field is moderately advanced, with companies like DuPont de Nemours, Inc. and Wanhua Chemical Group Co., Ltd. contributing to ongoing developments. The competitive landscape is characterized by a mix of large petrochemical corporations and specialized research institutions, each bringing unique expertise to address the challenges of extreme temperature stability in 2-Methylpentane.
Mitsui Chemicals, Inc.
Technical Solution: Mitsui Chemicals has conducted comprehensive stability analysis of 2-methylpentane using a combination of experimental and computational methods. Their approach includes thermo-gravimetric analysis (TGA) coupled with mass spectrometry to identify decomposition products at high temperatures[4]. They have also employed density functional theory (DFT) calculations to predict the molecular behavior of 2-methylpentane under extreme conditions[5]. Mitsui's research has led to the development of novel stabilizers that significantly improve the thermal resistance of 2-methylpentane in polymer applications, particularly in high-temperature resistant plastics[6].
Strengths: Strong integration of experimental and computational methods, focus on practical applications in polymer industry. Weaknesses: May have less emphasis on low-temperature stability analysis compared to high-temperature studies.
Wanhua Chemical Group Co., Ltd.
Technical Solution: Wanhua Chemical has focused on the stability analysis of 2-methylpentane in the context of its use in polyurethane foam production. Their approach involves using differential thermal analysis (DTA) and thermomechanical analysis (TMA) to study the compound's behavior under processing conditions[10]. They have developed a proprietary method for stabilizing 2-methylpentane-based blowing agents, allowing for improved thermal resistance in rigid polyurethane foams[11]. Wanhua's research has also explored the use of nanoparticle additives to enhance the overall stability and performance of 2-methylpentane in extreme temperature applications[12].
Strengths: Specialized knowledge in polyurethane applications, innovative use of nanoparticle technology. Weaknesses: Research may be narrowly focused on foam-related applications, potentially limiting broader chemical stability insights.
Core Technical Insights
4-methyl-1-pentene polymer
PatentPendingEP4303239A1
Innovation
- A 4-methyl-1-pentene polymer copolymerized with linear α-olefins, having specific melting and crystallization temperatures, and intrinsic viscosity ranges to ensure excellent heat resistance, solubility, and coating appearance when stretched or bended, while maintaining storage stability.
High thermal stability thermal cutoff device pellet composition
PatentWO2016033722A1
Innovation
- Use of an organic compound with low ionization potential, such as dibenzosuberenone, to enhance thermal stability of the pellet composition.
- Improved overshoot temperature ranges, with a maximum dielectric capability temperature (Tcap) at least 160°C greater than the transition temperature (Tf).
- Enhanced processing and pelletizing methods for improved aging performance of the thermal cutoff device.
Safety Regulations
The safety regulations surrounding the stability analysis of 2-methylpentane in extreme temperature conditions are crucial for ensuring the safe handling, storage, and transportation of this volatile organic compound. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) have established stringent guidelines for working with flammable liquids like 2-methylpentane.
OSHA's Hazard Communication Standard (29 CFR 1910.1200) mandates that all employers provide information about the hazards and identities of chemicals their workers are exposed to. This includes proper labeling, safety data sheets, and employee training programs specific to 2-methylpentane's properties under extreme temperatures. The standard also requires employers to implement engineering controls and work practices to minimize employee exposure.
The EPA regulates 2-methylpentane under the Toxic Substances Control Act (TSCA) and the Clean Air Act. These regulations focus on preventing environmental release and managing potential risks associated with its production and use, especially under varying temperature conditions. Facilities handling 2-methylpentane must comply with EPA's Risk Management Program (40 CFR Part 68) if they exceed threshold quantities.
International regulations, such as the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, also apply to 2-methylpentane. REACH requires companies to register chemical substances and provide safety information, including stability data under extreme conditions.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under GHS, 2-methylpentane is classified as a flammable liquid (Category 2), and its safety data sheet must include information on stability and reactivity under various environmental conditions.
Specific to extreme temperature conditions, regulations require comprehensive stability testing and documentation. ASTM International's standard test methods, such as ASTM E537 for thermal stability, provide guidelines for assessing chemical stability at high temperatures. For low-temperature stability, methods like ASTM D2386 for freezing point of aviation fuels may be adapted.
Transportation of 2-methylpentane is regulated by the Department of Transportation's Hazardous Materials Regulations (49 CFR Parts 171-180). These regulations specify requirements for packaging, labeling, and shipping papers, with particular attention to temperature control during transport to prevent instability or phase changes.
Compliance with these regulations necessitates ongoing monitoring and documentation of 2-methylpentane's stability under the full range of anticipated temperature conditions. This includes implementing robust quality control measures, regular equipment inspections, and emergency response plans tailored to potential stability-related incidents at extreme temperatures.
OSHA's Hazard Communication Standard (29 CFR 1910.1200) mandates that all employers provide information about the hazards and identities of chemicals their workers are exposed to. This includes proper labeling, safety data sheets, and employee training programs specific to 2-methylpentane's properties under extreme temperatures. The standard also requires employers to implement engineering controls and work practices to minimize employee exposure.
The EPA regulates 2-methylpentane under the Toxic Substances Control Act (TSCA) and the Clean Air Act. These regulations focus on preventing environmental release and managing potential risks associated with its production and use, especially under varying temperature conditions. Facilities handling 2-methylpentane must comply with EPA's Risk Management Program (40 CFR Part 68) if they exceed threshold quantities.
International regulations, such as the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, also apply to 2-methylpentane. REACH requires companies to register chemical substances and provide safety information, including stability data under extreme conditions.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under GHS, 2-methylpentane is classified as a flammable liquid (Category 2), and its safety data sheet must include information on stability and reactivity under various environmental conditions.
Specific to extreme temperature conditions, regulations require comprehensive stability testing and documentation. ASTM International's standard test methods, such as ASTM E537 for thermal stability, provide guidelines for assessing chemical stability at high temperatures. For low-temperature stability, methods like ASTM D2386 for freezing point of aviation fuels may be adapted.
Transportation of 2-methylpentane is regulated by the Department of Transportation's Hazardous Materials Regulations (49 CFR Parts 171-180). These regulations specify requirements for packaging, labeling, and shipping papers, with particular attention to temperature control during transport to prevent instability or phase changes.
Compliance with these regulations necessitates ongoing monitoring and documentation of 2-methylpentane's stability under the full range of anticipated temperature conditions. This includes implementing robust quality control measures, regular equipment inspections, and emergency response plans tailored to potential stability-related incidents at extreme temperatures.
Environmental Impact
The environmental impact of 2-methylpentane stability analysis in extreme temperature conditions is a critical consideration for both research and industrial applications. This isomeric hydrocarbon, commonly used in various industrial processes, poses potential risks to the environment when subjected to extreme temperatures.
At high temperatures, 2-methylpentane may undergo thermal decomposition, releasing volatile organic compounds (VOCs) into the atmosphere. These VOCs can contribute to the formation of ground-level ozone, a major component of smog, which can have detrimental effects on air quality and human health. Additionally, the release of these compounds may lead to the depletion of stratospheric ozone, exacerbating the greenhouse effect and contributing to global warming.
In extreme cold conditions, the reduced volatility of 2-methylpentane may result in its accumulation in soil and water bodies. This accumulation can potentially disrupt local ecosystems, affecting plant growth and aquatic life. The persistence of 2-methylpentane in the environment under low temperatures may also lead to long-term contamination of groundwater resources.
The stability analysis of 2-methylpentane in extreme temperature conditions also has implications for waste management and disposal practices. Improper handling or disposal of this compound, especially in areas experiencing temperature extremes, may result in soil and water pollution. This contamination can have far-reaching consequences for local flora and fauna, potentially disrupting food chains and biodiversity.
From a broader perspective, the energy requirements for maintaining extreme temperature conditions during stability analysis contribute to increased carbon emissions. This indirect environmental impact should be considered when assessing the overall ecological footprint of research and industrial processes involving 2-methylpentane.
To mitigate these environmental risks, it is crucial to implement robust containment and handling protocols during stability analysis experiments. The use of closed-loop systems, efficient filtration technologies, and proper waste management practices can significantly reduce the potential for environmental contamination. Furthermore, the development of more environmentally friendly alternatives or the optimization of processes to minimize the use of 2-methylpentane should be explored as part of a comprehensive sustainability strategy.
In conclusion, while the stability analysis of 2-methylpentane in extreme temperature conditions is essential for various applications, it is imperative to carefully consider and address the potential environmental impacts. By adopting responsible research practices and investing in eco-friendly technologies, the scientific community can strike a balance between advancing knowledge and preserving the environment for future generations.
At high temperatures, 2-methylpentane may undergo thermal decomposition, releasing volatile organic compounds (VOCs) into the atmosphere. These VOCs can contribute to the formation of ground-level ozone, a major component of smog, which can have detrimental effects on air quality and human health. Additionally, the release of these compounds may lead to the depletion of stratospheric ozone, exacerbating the greenhouse effect and contributing to global warming.
In extreme cold conditions, the reduced volatility of 2-methylpentane may result in its accumulation in soil and water bodies. This accumulation can potentially disrupt local ecosystems, affecting plant growth and aquatic life. The persistence of 2-methylpentane in the environment under low temperatures may also lead to long-term contamination of groundwater resources.
The stability analysis of 2-methylpentane in extreme temperature conditions also has implications for waste management and disposal practices. Improper handling or disposal of this compound, especially in areas experiencing temperature extremes, may result in soil and water pollution. This contamination can have far-reaching consequences for local flora and fauna, potentially disrupting food chains and biodiversity.
From a broader perspective, the energy requirements for maintaining extreme temperature conditions during stability analysis contribute to increased carbon emissions. This indirect environmental impact should be considered when assessing the overall ecological footprint of research and industrial processes involving 2-methylpentane.
To mitigate these environmental risks, it is crucial to implement robust containment and handling protocols during stability analysis experiments. The use of closed-loop systems, efficient filtration technologies, and proper waste management practices can significantly reduce the potential for environmental contamination. Furthermore, the development of more environmentally friendly alternatives or the optimization of processes to minimize the use of 2-methylpentane should be explored as part of a comprehensive sustainability strategy.
In conclusion, while the stability analysis of 2-methylpentane in extreme temperature conditions is essential for various applications, it is imperative to carefully consider and address the potential environmental impacts. By adopting responsible research practices and investing in eco-friendly technologies, the scientific community can strike a balance between advancing knowledge and preserving the environment for future generations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!