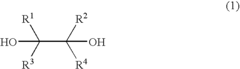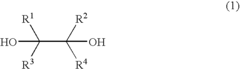Advanced Methods for Carbonyl Compound Isolation
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Isolation Background and Objectives
Carbonyl compounds play a crucial role in organic chemistry and various industrial applications. The isolation and purification of these compounds have been a subject of extensive research and development over the past century. The evolution of carbonyl isolation techniques has been driven by the need for more efficient, selective, and environmentally friendly methods to meet the growing demands of pharmaceutical, agrochemical, and fine chemical industries.
The primary objective of advanced carbonyl compound isolation methods is to achieve high purity and yield while minimizing the use of harmful solvents and reducing energy consumption. Traditional methods, such as distillation and crystallization, have been widely used but often face limitations in terms of efficiency and applicability to complex mixtures. This has led to the development of more sophisticated techniques that leverage the unique chemical properties of carbonyl compounds.
One of the key drivers for innovation in this field has been the increasing emphasis on green chemistry principles. Researchers and industry professionals are focusing on developing sustainable isolation processes that reduce waste generation and utilize renewable resources. This shift has resulted in the exploration of novel approaches such as supercritical fluid extraction, membrane-based separations, and bio-inspired separation techniques.
The technological evolution in carbonyl isolation has also been influenced by advancements in analytical instrumentation. High-resolution chromatography, mass spectrometry, and spectroscopic methods have enabled more precise identification and quantification of carbonyl compounds in complex matrices. This has, in turn, facilitated the development of more targeted and efficient isolation strategies.
Another significant trend in the field is the integration of computational methods and artificial intelligence to optimize isolation processes. Machine learning algorithms are being employed to predict the most suitable isolation conditions for specific carbonyl compounds, leading to more rapid and cost-effective method development.
As we look towards the future, the goals for advanced carbonyl isolation methods are centered around achieving higher selectivity, improved scalability, and enhanced sustainability. There is a growing interest in developing multi-functional materials that can selectively capture and release carbonyl compounds under mild conditions. Additionally, the integration of continuous flow processes and process intensification techniques is expected to revolutionize large-scale isolation of carbonyl compounds in industrial settings.
The primary objective of advanced carbonyl compound isolation methods is to achieve high purity and yield while minimizing the use of harmful solvents and reducing energy consumption. Traditional methods, such as distillation and crystallization, have been widely used but often face limitations in terms of efficiency and applicability to complex mixtures. This has led to the development of more sophisticated techniques that leverage the unique chemical properties of carbonyl compounds.
One of the key drivers for innovation in this field has been the increasing emphasis on green chemistry principles. Researchers and industry professionals are focusing on developing sustainable isolation processes that reduce waste generation and utilize renewable resources. This shift has resulted in the exploration of novel approaches such as supercritical fluid extraction, membrane-based separations, and bio-inspired separation techniques.
The technological evolution in carbonyl isolation has also been influenced by advancements in analytical instrumentation. High-resolution chromatography, mass spectrometry, and spectroscopic methods have enabled more precise identification and quantification of carbonyl compounds in complex matrices. This has, in turn, facilitated the development of more targeted and efficient isolation strategies.
Another significant trend in the field is the integration of computational methods and artificial intelligence to optimize isolation processes. Machine learning algorithms are being employed to predict the most suitable isolation conditions for specific carbonyl compounds, leading to more rapid and cost-effective method development.
As we look towards the future, the goals for advanced carbonyl isolation methods are centered around achieving higher selectivity, improved scalability, and enhanced sustainability. There is a growing interest in developing multi-functional materials that can selectively capture and release carbonyl compounds under mild conditions. Additionally, the integration of continuous flow processes and process intensification techniques is expected to revolutionize large-scale isolation of carbonyl compounds in industrial settings.
Market Analysis for Carbonyl Compound Applications
The market for carbonyl compound applications has shown significant growth and diversification in recent years, driven by increasing demand across various industries. Carbonyl compounds, characterized by their C=O functional group, play a crucial role in numerous chemical processes and end-products, making them essential in sectors such as pharmaceuticals, agrochemicals, flavors and fragrances, and materials science.
In the pharmaceutical industry, carbonyl compounds serve as key intermediates in the synthesis of many active pharmaceutical ingredients (APIs). The global pharmaceutical market, valued at over $1.4 trillion in 2021, is expected to continue its growth trajectory, thereby fueling the demand for carbonyl compounds. Particularly, the development of novel drugs and the increasing focus on personalized medicine are creating new opportunities for specialized carbonyl compounds.
The agrochemical sector represents another significant market for carbonyl compounds, especially in the production of pesticides and herbicides. With the global population projected to reach 9.7 billion by 2050, the pressure on agricultural productivity is intensifying, driving the demand for more effective crop protection solutions. This trend is expected to sustain the growth of carbonyl compound applications in agrochemicals.
The flavors and fragrances industry, valued at approximately $30 billion globally, heavily relies on carbonyl compounds for creating a wide range of aromas and tastes. Consumer preferences for natural and organic products are pushing manufacturers to develop new, nature-identical compounds, many of which are carbonyl-based. This shift is opening up new avenues for innovation and market expansion in the flavor and fragrance sector.
In materials science, carbonyl compounds are finding increasing applications in the development of advanced polymers, coatings, and adhesives. The global smart coatings market, for instance, is projected to grow at a CAGR of over 20% through 2026, with carbonyl-based compounds playing a crucial role in enhancing the performance and functionality of these materials.
The market for carbonyl compounds is also being shaped by sustainability concerns and regulatory pressures. There is a growing emphasis on developing greener synthesis methods and more environmentally friendly carbonyl compounds. This trend is driving research into bio-based carbonyl compounds and more efficient isolation techniques, aligning with the broader shift towards sustainable chemistry.
Geographically, Asia-Pacific is emerging as a key growth region for carbonyl compound applications, driven by rapid industrialization, increasing R&D investments, and growing end-user industries. North America and Europe continue to be significant markets, particularly for high-value, specialized carbonyl compounds used in pharmaceuticals and advanced materials.
In the pharmaceutical industry, carbonyl compounds serve as key intermediates in the synthesis of many active pharmaceutical ingredients (APIs). The global pharmaceutical market, valued at over $1.4 trillion in 2021, is expected to continue its growth trajectory, thereby fueling the demand for carbonyl compounds. Particularly, the development of novel drugs and the increasing focus on personalized medicine are creating new opportunities for specialized carbonyl compounds.
The agrochemical sector represents another significant market for carbonyl compounds, especially in the production of pesticides and herbicides. With the global population projected to reach 9.7 billion by 2050, the pressure on agricultural productivity is intensifying, driving the demand for more effective crop protection solutions. This trend is expected to sustain the growth of carbonyl compound applications in agrochemicals.
The flavors and fragrances industry, valued at approximately $30 billion globally, heavily relies on carbonyl compounds for creating a wide range of aromas and tastes. Consumer preferences for natural and organic products are pushing manufacturers to develop new, nature-identical compounds, many of which are carbonyl-based. This shift is opening up new avenues for innovation and market expansion in the flavor and fragrance sector.
In materials science, carbonyl compounds are finding increasing applications in the development of advanced polymers, coatings, and adhesives. The global smart coatings market, for instance, is projected to grow at a CAGR of over 20% through 2026, with carbonyl-based compounds playing a crucial role in enhancing the performance and functionality of these materials.
The market for carbonyl compounds is also being shaped by sustainability concerns and regulatory pressures. There is a growing emphasis on developing greener synthesis methods and more environmentally friendly carbonyl compounds. This trend is driving research into bio-based carbonyl compounds and more efficient isolation techniques, aligning with the broader shift towards sustainable chemistry.
Geographically, Asia-Pacific is emerging as a key growth region for carbonyl compound applications, driven by rapid industrialization, increasing R&D investments, and growing end-user industries. North America and Europe continue to be significant markets, particularly for high-value, specialized carbonyl compounds used in pharmaceuticals and advanced materials.
Current Challenges in Carbonyl Isolation Techniques
The isolation of carbonyl compounds presents several significant challenges in modern analytical chemistry and organic synthesis. One of the primary difficulties lies in the reactivity of carbonyl groups, which can easily undergo unwanted side reactions during isolation processes. This reactivity often leads to the formation of byproducts or degradation of the target compounds, compromising the purity and yield of the isolated carbonyls.
Another major challenge is the volatility of many carbonyl compounds, particularly aldehydes and ketones with low molecular weights. These compounds can easily evaporate during conventional isolation techniques, resulting in substantial losses and reduced recovery rates. This issue is particularly problematic when dealing with trace amounts of carbonyl compounds in complex matrices.
The presence of structurally similar compounds in mixtures poses an additional challenge for carbonyl isolation. Many separation techniques struggle to achieve high selectivity between carbonyl compounds and other functionalities, such as alcohols or carboxylic acids, which can have similar physical properties. This lack of selectivity often necessitates multiple purification steps, increasing the complexity and duration of the isolation process.
Water solubility is another factor that complicates the isolation of carbonyl compounds, especially in environmental and biological samples. Many carbonyl compounds exhibit significant water solubility, making their extraction from aqueous matrices difficult without the use of specialized techniques or derivatization methods. This challenge is particularly relevant in the analysis of atmospheric and water samples for carbonyl pollutants.
The stability of carbonyl compounds under various isolation conditions also presents a significant hurdle. Many carbonyls are sensitive to pH changes, light exposure, or elevated temperatures, which are common factors in traditional isolation methods. This sensitivity can lead to isomerization, decomposition, or polymerization of the target compounds during the isolation process, affecting the accuracy of subsequent analyses.
Furthermore, the wide range of carbonyl compound polarities and molecular weights makes it challenging to develop a universal isolation method. Techniques that work well for small, volatile aldehydes may be ineffective for larger, less volatile ketones or complex carbonyl-containing biomolecules. This diversity necessitates the development of multiple specialized techniques, each tailored to specific types of carbonyl compounds.
Lastly, the presence of interfering substances in complex matrices, such as biological samples or environmental specimens, can significantly hinder the isolation of carbonyl compounds. These interferents may co-elute with the target carbonyls or react with them during the isolation process, necessitating additional purification steps or the development of highly selective extraction methods.
Another major challenge is the volatility of many carbonyl compounds, particularly aldehydes and ketones with low molecular weights. These compounds can easily evaporate during conventional isolation techniques, resulting in substantial losses and reduced recovery rates. This issue is particularly problematic when dealing with trace amounts of carbonyl compounds in complex matrices.
The presence of structurally similar compounds in mixtures poses an additional challenge for carbonyl isolation. Many separation techniques struggle to achieve high selectivity between carbonyl compounds and other functionalities, such as alcohols or carboxylic acids, which can have similar physical properties. This lack of selectivity often necessitates multiple purification steps, increasing the complexity and duration of the isolation process.
Water solubility is another factor that complicates the isolation of carbonyl compounds, especially in environmental and biological samples. Many carbonyl compounds exhibit significant water solubility, making their extraction from aqueous matrices difficult without the use of specialized techniques or derivatization methods. This challenge is particularly relevant in the analysis of atmospheric and water samples for carbonyl pollutants.
The stability of carbonyl compounds under various isolation conditions also presents a significant hurdle. Many carbonyls are sensitive to pH changes, light exposure, or elevated temperatures, which are common factors in traditional isolation methods. This sensitivity can lead to isomerization, decomposition, or polymerization of the target compounds during the isolation process, affecting the accuracy of subsequent analyses.
Furthermore, the wide range of carbonyl compound polarities and molecular weights makes it challenging to develop a universal isolation method. Techniques that work well for small, volatile aldehydes may be ineffective for larger, less volatile ketones or complex carbonyl-containing biomolecules. This diversity necessitates the development of multiple specialized techniques, each tailored to specific types of carbonyl compounds.
Lastly, the presence of interfering substances in complex matrices, such as biological samples or environmental specimens, can significantly hinder the isolation of carbonyl compounds. These interferents may co-elute with the target carbonyls or react with them during the isolation process, necessitating additional purification steps or the development of highly selective extraction methods.
State-of-the-Art Carbonyl Isolation Approaches
01 Chromatographic separation techniques
Various chromatographic methods are employed for the isolation of carbonyl compounds. These techniques include liquid chromatography, gas chromatography, and high-performance liquid chromatography (HPLC). These methods allow for the separation and purification of carbonyl compounds based on their physical and chemical properties, enabling efficient isolation from complex mixtures.- Chromatographic separation techniques: Various chromatographic methods are employed for the isolation of carbonyl compounds. These techniques include liquid chromatography, gas chromatography, and high-performance liquid chromatography (HPLC). These methods allow for the separation and purification of carbonyl compounds based on their physical and chemical properties, enabling efficient isolation from complex mixtures.
- Derivatization and extraction methods: Carbonyl compounds can be isolated through derivatization followed by extraction. This process involves converting the carbonyl compounds into derivatives that are easier to isolate or detect. Common derivatization agents include 2,4-dinitrophenylhydrazine (DNPH) and o-phenylenediamine. After derivatization, the compounds can be extracted using organic solvents and further purified.
- Distillation and fractional distillation: Distillation techniques are used for isolating carbonyl compounds, especially when dealing with mixtures of compounds with different boiling points. Fractional distillation allows for the separation of compounds with similar boiling points. These methods are particularly useful for isolating volatile carbonyl compounds from less volatile substances.
- Solid-phase extraction (SPE): Solid-phase extraction is a technique used to isolate carbonyl compounds from liquid samples. This method involves passing the sample through a solid adsorbent material that selectively retains the carbonyl compounds. The compounds are then eluted from the adsorbent using an appropriate solvent. SPE offers advantages such as high recovery rates and the ability to concentrate samples.
- Microextraction techniques: Microextraction methods, such as solid-phase microextraction (SPME) and liquid-phase microextraction (LPME), are used for isolating carbonyl compounds from various matrices. These techniques involve extracting the compounds using a small amount of extracting phase, either solid or liquid. Microextraction methods are particularly useful for trace analysis and can be coupled with various analytical instruments for detection and quantification.
02 Derivatization methods
Carbonyl compounds can be isolated through derivatization techniques. This involves converting the carbonyl compounds into derivatives that are easier to separate or detect. Common derivatization agents include 2,4-dinitrophenylhydrazine (DNPH) and o-phenylenediamine. These methods enhance the selectivity and sensitivity of the isolation process.Expand Specific Solutions03 Extraction and distillation processes
Extraction methods, such as liquid-liquid extraction or solid-phase extraction, are used to isolate carbonyl compounds from various matrices. Additionally, distillation techniques, including fractional distillation and steam distillation, can be employed for the separation of carbonyl compounds based on their boiling points and vapor pressures.Expand Specific Solutions04 Selective adsorption and desorption
Carbonyl compounds can be isolated using selective adsorption materials, such as molecularly imprinted polymers (MIPs) or specific adsorbents. These materials selectively bind to the target carbonyl compounds, allowing for their separation from complex mixtures. The adsorbed compounds are then recovered through desorption processes.Expand Specific Solutions05 Enzymatic and biological methods
Enzymatic and biological approaches can be used for the isolation of carbonyl compounds. These methods utilize specific enzymes or microorganisms that selectively interact with or metabolize carbonyl compounds. Such techniques can offer high selectivity and efficiency in isolating target compounds from complex biological matrices.Expand Specific Solutions
Key Players in Carbonyl Isolation Technology
The field of advanced methods for carbonyl compound isolation is in a mature stage of development, with a competitive landscape dominated by established chemical and pharmaceutical companies. The market size is substantial, driven by applications in various industries including pharmaceuticals, agrochemicals, and materials science. Key players like BASF, Sumitomo Chemical, and China Petroleum & Chemical Corp. have significant market presence, leveraging their extensive R&D capabilities and global reach. The technology's maturity is evident in the involvement of academic institutions such as Nagoya University and Tianjin University, which contribute to ongoing research and innovation. Smaller specialized firms like Kainos Medicine and Yungjin Pharm also play important roles in developing niche applications and driving technological advancements in this field.
BASF Corp.
Technical Solution: BASF Corp. has developed advanced methods for carbonyl compound isolation using a combination of selective adsorption and membrane technology. Their approach involves a two-step process: first, carbonyl compounds are selectively adsorbed onto specially designed porous materials with tailored surface chemistry[1]. This is followed by a membrane separation step using advanced polymer membranes with high selectivity for carbonyl compounds[2]. The process achieves high purity (>99%) and recovery rates (>95%) for a wide range of carbonyl compounds, including aldehydes and ketones[3]. BASF has also incorporated green chemistry principles, using bio-based solvents and minimizing waste generation in the isolation process[4].
Strengths: High purity and recovery rates, versatility for various carbonyl compounds, environmentally friendly approach. Weaknesses: Potentially higher initial investment costs, may require specialized equipment and expertise.
Kureha Corp.
Technical Solution: Kureha Corp. has pioneered a novel approach to carbonyl compound isolation using supercritical fluid extraction (SFE) technology. Their method employs supercritical CO2 as the primary extraction medium, which is modified with carefully selected co-solvents to enhance selectivity for carbonyl compounds[1]. The process operates at moderate temperatures (40-60°C) and pressures (100-300 bar), allowing for gentle extraction of sensitive carbonyl compounds[2]. Kureha's technology incorporates in-line fractionation columns to separate different carbonyl species based on their molecular weight and polarity[3]. This approach enables the isolation of high-purity carbonyl compounds (>98%) with minimal thermal degradation and solvent residues[4].
Strengths: Gentle extraction conditions, high purity, minimal solvent usage. Weaknesses: High-pressure equipment required, potential scalability challenges for large-volume production.
Innovative Patents in Carbonyl Compound Separation
Polymeric reagents for the isolation and protection of carbonyl compounds
PatentInactiveUS4461876A
Innovation
- A polymeric reagent with aminooxy functional groups (--ONH2) is developed, which reacts with carbonyl compounds to form oximes, allowing for efficient binding and subsequent mild hydrolysis for release, overcoming the limitations of existing Girard reagents and polymer-bound hydrazide reagents.
Process for production of carbonyl compounds
PatentInactiveUS20060089506A1
Innovation
- A process involving the reaction of a diol with bromine or an inorganic bromine compound in the presence of a trivalent bismuth compound and a base to produce carbonyl compounds, which includes using a diol represented by formula (1) with specific substituents and a trivalent bismuth compound like triphenylbismuth, along with a base such as potassium carbonate, to form carbonyl compounds like aldehydes.
Environmental Impact of Carbonyl Isolation Processes
The environmental impact of carbonyl isolation processes is a critical consideration in the development and implementation of advanced methods for carbonyl compound isolation. Traditional isolation techniques often involve the use of organic solvents and energy-intensive processes, which can have significant environmental consequences. These impacts include air and water pollution, greenhouse gas emissions, and the generation of hazardous waste.
Recent advancements in carbonyl isolation methods have focused on reducing these environmental impacts. Green chemistry principles are increasingly being applied to develop more sustainable isolation processes. One approach involves the use of supercritical carbon dioxide (scCO2) as an alternative solvent. scCO2 is non-toxic, non-flammable, and can be easily recycled, making it an environmentally friendly option. This method has shown promise in reducing the carbon footprint of carbonyl isolation processes.
Another environmentally conscious approach is the development of solvent-free isolation techniques. These methods, such as mechanochemical processes and solid-state reactions, eliminate the need for organic solvents altogether. By reducing solvent usage, these techniques not only minimize waste generation but also decrease energy consumption associated with solvent recovery and purification.
Biocatalytic methods for carbonyl isolation have also gained attention due to their potential for reduced environmental impact. Enzymes can catalyze highly selective reactions under mild conditions, often in aqueous media. This approach can significantly reduce energy requirements and minimize the use of harsh chemicals, leading to a more environmentally benign process.
The implementation of continuous flow processes for carbonyl isolation has shown potential for improving environmental performance. These systems often require smaller reaction volumes, leading to reduced solvent consumption and waste generation. Additionally, continuous flow processes can be more energy-efficient than batch processes, further reducing the overall environmental footprint.
Life cycle assessment (LCA) studies have been conducted to quantify the environmental impacts of various carbonyl isolation methods. These assessments consider factors such as raw material extraction, energy consumption, waste generation, and end-of-life disposal. LCA results have highlighted the importance of considering the entire process lifecycle when evaluating environmental impacts, rather than focusing solely on the isolation step.
As regulations on environmental protection become more stringent, there is an increasing emphasis on developing carbonyl isolation processes that meet both technical and environmental requirements. This has led to the exploration of novel materials and technologies, such as ionic liquids and membrane-based separation processes, which offer potential environmental benefits over conventional methods.
Recent advancements in carbonyl isolation methods have focused on reducing these environmental impacts. Green chemistry principles are increasingly being applied to develop more sustainable isolation processes. One approach involves the use of supercritical carbon dioxide (scCO2) as an alternative solvent. scCO2 is non-toxic, non-flammable, and can be easily recycled, making it an environmentally friendly option. This method has shown promise in reducing the carbon footprint of carbonyl isolation processes.
Another environmentally conscious approach is the development of solvent-free isolation techniques. These methods, such as mechanochemical processes and solid-state reactions, eliminate the need for organic solvents altogether. By reducing solvent usage, these techniques not only minimize waste generation but also decrease energy consumption associated with solvent recovery and purification.
Biocatalytic methods for carbonyl isolation have also gained attention due to their potential for reduced environmental impact. Enzymes can catalyze highly selective reactions under mild conditions, often in aqueous media. This approach can significantly reduce energy requirements and minimize the use of harsh chemicals, leading to a more environmentally benign process.
The implementation of continuous flow processes for carbonyl isolation has shown potential for improving environmental performance. These systems often require smaller reaction volumes, leading to reduced solvent consumption and waste generation. Additionally, continuous flow processes can be more energy-efficient than batch processes, further reducing the overall environmental footprint.
Life cycle assessment (LCA) studies have been conducted to quantify the environmental impacts of various carbonyl isolation methods. These assessments consider factors such as raw material extraction, energy consumption, waste generation, and end-of-life disposal. LCA results have highlighted the importance of considering the entire process lifecycle when evaluating environmental impacts, rather than focusing solely on the isolation step.
As regulations on environmental protection become more stringent, there is an increasing emphasis on developing carbonyl isolation processes that meet both technical and environmental requirements. This has led to the exploration of novel materials and technologies, such as ionic liquids and membrane-based separation processes, which offer potential environmental benefits over conventional methods.
Regulatory Framework for Carbonyl Compound Handling
The regulatory framework for carbonyl compound handling is a critical aspect of ensuring safety and compliance in research, industrial, and environmental settings. Governments and international organizations have established comprehensive guidelines and regulations to address the potential hazards associated with these compounds.
At the forefront of regulatory efforts is the Occupational Safety and Health Administration (OSHA) in the United States. OSHA has set specific permissible exposure limits (PELs) for various carbonyl compounds, including formaldehyde, acetaldehyde, and acrolein. These limits are designed to protect workers from adverse health effects associated with long-term exposure. Additionally, OSHA mandates the use of personal protective equipment (PPE) and engineering controls to minimize exposure risks.
The Environmental Protection Agency (EPA) plays a crucial role in regulating carbonyl compounds under the Clean Air Act and the Toxic Substances Control Act. The EPA has established National Emission Standards for Hazardous Air Pollutants (NESHAP) that specifically address carbonyl emissions from industrial sources. These standards require the implementation of Maximum Achievable Control Technology (MACT) to reduce emissions to the lowest possible levels.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the handling and use of carbonyl compounds. REACH requires manufacturers and importers to register substances and provide detailed safety information. The Classification, Labeling, and Packaging (CLP) Regulation complements REACH by ensuring that hazards are clearly communicated to workers and consumers.
The International Agency for Research on Cancer (IARC) has classified several carbonyl compounds, such as formaldehyde, as carcinogenic to humans. This classification has led to stricter regulations and increased scrutiny of these compounds in various industries. As a result, many countries have implemented more stringent exposure limits and monitoring requirements.
In the context of advanced methods for carbonyl compound isolation, regulatory frameworks often require the use of best available techniques (BAT) to minimize environmental impact and worker exposure. This includes the implementation of closed-loop systems, advanced filtration technologies, and real-time monitoring of air quality in work environments.
Regulatory bodies also emphasize the importance of proper waste management and disposal of carbonyl compounds. Many countries have specific regulations governing the transportation, storage, and disposal of these substances, often requiring specialized handling procedures and documentation.
As research into advanced isolation methods progresses, regulatory frameworks are evolving to keep pace with new technologies. There is an increasing focus on promoting green chemistry principles and encouraging the development of safer alternatives to traditional carbonyl compounds. This shift is reflected in updated regulations that incentivize the use of less hazardous substances and more environmentally friendly isolation techniques.
At the forefront of regulatory efforts is the Occupational Safety and Health Administration (OSHA) in the United States. OSHA has set specific permissible exposure limits (PELs) for various carbonyl compounds, including formaldehyde, acetaldehyde, and acrolein. These limits are designed to protect workers from adverse health effects associated with long-term exposure. Additionally, OSHA mandates the use of personal protective equipment (PPE) and engineering controls to minimize exposure risks.
The Environmental Protection Agency (EPA) plays a crucial role in regulating carbonyl compounds under the Clean Air Act and the Toxic Substances Control Act. The EPA has established National Emission Standards for Hazardous Air Pollutants (NESHAP) that specifically address carbonyl emissions from industrial sources. These standards require the implementation of Maximum Achievable Control Technology (MACT) to reduce emissions to the lowest possible levels.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the handling and use of carbonyl compounds. REACH requires manufacturers and importers to register substances and provide detailed safety information. The Classification, Labeling, and Packaging (CLP) Regulation complements REACH by ensuring that hazards are clearly communicated to workers and consumers.
The International Agency for Research on Cancer (IARC) has classified several carbonyl compounds, such as formaldehyde, as carcinogenic to humans. This classification has led to stricter regulations and increased scrutiny of these compounds in various industries. As a result, many countries have implemented more stringent exposure limits and monitoring requirements.
In the context of advanced methods for carbonyl compound isolation, regulatory frameworks often require the use of best available techniques (BAT) to minimize environmental impact and worker exposure. This includes the implementation of closed-loop systems, advanced filtration technologies, and real-time monitoring of air quality in work environments.
Regulatory bodies also emphasize the importance of proper waste management and disposal of carbonyl compounds. Many countries have specific regulations governing the transportation, storage, and disposal of these substances, often requiring specialized handling procedures and documentation.
As research into advanced isolation methods progresses, regulatory frameworks are evolving to keep pace with new technologies. There is an increasing focus on promoting green chemistry principles and encouraging the development of safer alternatives to traditional carbonyl compounds. This shift is reflected in updated regulations that incentivize the use of less hazardous substances and more environmentally friendly isolation techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!