Exploring Carbonyl Group Techniques for Optimized Efficiency
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Group Research Background and Objectives
Carbonyl groups have been a focal point of organic chemistry research for decades, playing a crucial role in numerous chemical reactions and industrial processes. The exploration of carbonyl group techniques for optimized efficiency has gained significant momentum in recent years, driven by the need for more sustainable and cost-effective chemical processes across various industries.
The study of carbonyl groups dates back to the early 19th century when chemists first identified and characterized these functional groups. Since then, our understanding of carbonyl chemistry has evolved dramatically, leading to groundbreaking discoveries in fields such as pharmaceuticals, materials science, and energy production. The unique reactivity of carbonyl groups, stemming from their polarized carbon-oxygen double bond, has made them invaluable in synthetic organic chemistry and industrial applications.
In the context of optimizing efficiency, research on carbonyl group techniques has focused on several key areas. One primary objective is to develop more selective and energy-efficient methods for carbonyl group transformations. This includes the exploration of novel catalysts and reaction conditions that can facilitate carbonyl reactions with higher yields and fewer side products. Additionally, researchers are investigating ways to harness the reactivity of carbonyl groups in green chemistry applications, aiming to reduce the environmental impact of chemical processes.
Another significant aspect of carbonyl group research is the development of new synthetic methodologies. Scientists are continually seeking innovative approaches to form and manipulate carbonyl groups, with a particular emphasis on atom-economic reactions and one-pot syntheses. These efforts aim to streamline chemical processes, reduce waste, and improve overall efficiency in both laboratory and industrial settings.
The advent of computational chemistry has also greatly influenced carbonyl group research. Advanced modeling techniques now allow researchers to predict reaction outcomes and design more efficient synthetic routes. This computational approach, combined with experimental studies, has accelerated the discovery of new carbonyl-based materials and reaction pathways.
As we look to the future, the objectives of carbonyl group research are becoming increasingly aligned with global challenges. There is a growing focus on utilizing carbonyl chemistry in the development of sustainable materials, such as biodegradable polymers and environmentally friendly solvents. Furthermore, researchers are exploring the potential of carbonyl compounds in energy storage and conversion technologies, particularly in the context of renewable energy systems.
In conclusion, the exploration of carbonyl group techniques for optimized efficiency represents a dynamic and evolving field of study. By building on the rich history of carbonyl chemistry and leveraging cutting-edge technologies, researchers aim to unlock new possibilities for more efficient, sustainable, and versatile chemical processes. The ongoing advancements in this area promise to have far-reaching impacts across multiple industries and scientific disciplines.
The study of carbonyl groups dates back to the early 19th century when chemists first identified and characterized these functional groups. Since then, our understanding of carbonyl chemistry has evolved dramatically, leading to groundbreaking discoveries in fields such as pharmaceuticals, materials science, and energy production. The unique reactivity of carbonyl groups, stemming from their polarized carbon-oxygen double bond, has made them invaluable in synthetic organic chemistry and industrial applications.
In the context of optimizing efficiency, research on carbonyl group techniques has focused on several key areas. One primary objective is to develop more selective and energy-efficient methods for carbonyl group transformations. This includes the exploration of novel catalysts and reaction conditions that can facilitate carbonyl reactions with higher yields and fewer side products. Additionally, researchers are investigating ways to harness the reactivity of carbonyl groups in green chemistry applications, aiming to reduce the environmental impact of chemical processes.
Another significant aspect of carbonyl group research is the development of new synthetic methodologies. Scientists are continually seeking innovative approaches to form and manipulate carbonyl groups, with a particular emphasis on atom-economic reactions and one-pot syntheses. These efforts aim to streamline chemical processes, reduce waste, and improve overall efficiency in both laboratory and industrial settings.
The advent of computational chemistry has also greatly influenced carbonyl group research. Advanced modeling techniques now allow researchers to predict reaction outcomes and design more efficient synthetic routes. This computational approach, combined with experimental studies, has accelerated the discovery of new carbonyl-based materials and reaction pathways.
As we look to the future, the objectives of carbonyl group research are becoming increasingly aligned with global challenges. There is a growing focus on utilizing carbonyl chemistry in the development of sustainable materials, such as biodegradable polymers and environmentally friendly solvents. Furthermore, researchers are exploring the potential of carbonyl compounds in energy storage and conversion technologies, particularly in the context of renewable energy systems.
In conclusion, the exploration of carbonyl group techniques for optimized efficiency represents a dynamic and evolving field of study. By building on the rich history of carbonyl chemistry and leveraging cutting-edge technologies, researchers aim to unlock new possibilities for more efficient, sustainable, and versatile chemical processes. The ongoing advancements in this area promise to have far-reaching impacts across multiple industries and scientific disciplines.
Industrial Applications and Market Demand
The carbonyl group, a fundamental functional group in organic chemistry, has found extensive applications across various industries due to its unique reactivity and versatility. The market demand for carbonyl group-based products and technologies has been steadily growing, driven by advancements in chemical synthesis, materials science, and pharmaceutical research.
In the pharmaceutical industry, carbonyl group techniques play a crucial role in drug discovery and development. The ability to manipulate carbonyl groups allows for the creation of complex molecular structures, leading to the synthesis of novel therapeutic compounds. This has resulted in a significant market demand for carbonyl-based intermediates and reagents used in pharmaceutical manufacturing processes.
The polymer and materials science sectors have also witnessed increased interest in carbonyl group techniques. The incorporation of carbonyl functionalities into polymer chains has enabled the development of advanced materials with enhanced properties, such as improved adhesion, thermal stability, and chemical resistance. This has opened up new opportunities in industries ranging from automotive to aerospace, where high-performance materials are in constant demand.
In the field of organic electronics, carbonyl group techniques have gained traction for the synthesis of organic semiconductors and light-emitting materials. The market for organic light-emitting diodes (OLEDs) and organic photovoltaics has been expanding rapidly, driving the need for efficient carbonyl-based synthetic methods to produce key molecular components.
The food and beverage industry has also benefited from carbonyl group techniques, particularly in the development of flavors and fragrances. The ability to create complex aroma compounds through carbonyl chemistry has led to innovations in food additives and perfumery, meeting the growing consumer demand for diverse and natural-like sensory experiences.
Environmental applications of carbonyl group techniques have emerged as a promising market segment. The development of carbonyl-based adsorbents and catalysts for air and water purification has gained attention, addressing the increasing global focus on sustainability and pollution control.
The agrochemical sector has shown interest in carbonyl group techniques for the design of more effective and environmentally friendly pesticides and herbicides. This market demand is driven by the need for sustainable agricultural practices and stricter regulations on chemical usage in farming.
As industries continue to seek more efficient and sustainable processes, the demand for optimized carbonyl group techniques is expected to grow. This trend is further supported by the increasing emphasis on green chemistry principles and the need for atom-economical reactions in industrial settings.
In the pharmaceutical industry, carbonyl group techniques play a crucial role in drug discovery and development. The ability to manipulate carbonyl groups allows for the creation of complex molecular structures, leading to the synthesis of novel therapeutic compounds. This has resulted in a significant market demand for carbonyl-based intermediates and reagents used in pharmaceutical manufacturing processes.
The polymer and materials science sectors have also witnessed increased interest in carbonyl group techniques. The incorporation of carbonyl functionalities into polymer chains has enabled the development of advanced materials with enhanced properties, such as improved adhesion, thermal stability, and chemical resistance. This has opened up new opportunities in industries ranging from automotive to aerospace, where high-performance materials are in constant demand.
In the field of organic electronics, carbonyl group techniques have gained traction for the synthesis of organic semiconductors and light-emitting materials. The market for organic light-emitting diodes (OLEDs) and organic photovoltaics has been expanding rapidly, driving the need for efficient carbonyl-based synthetic methods to produce key molecular components.
The food and beverage industry has also benefited from carbonyl group techniques, particularly in the development of flavors and fragrances. The ability to create complex aroma compounds through carbonyl chemistry has led to innovations in food additives and perfumery, meeting the growing consumer demand for diverse and natural-like sensory experiences.
Environmental applications of carbonyl group techniques have emerged as a promising market segment. The development of carbonyl-based adsorbents and catalysts for air and water purification has gained attention, addressing the increasing global focus on sustainability and pollution control.
The agrochemical sector has shown interest in carbonyl group techniques for the design of more effective and environmentally friendly pesticides and herbicides. This market demand is driven by the need for sustainable agricultural practices and stricter regulations on chemical usage in farming.
As industries continue to seek more efficient and sustainable processes, the demand for optimized carbonyl group techniques is expected to grow. This trend is further supported by the increasing emphasis on green chemistry principles and the need for atom-economical reactions in industrial settings.
Current Challenges in Carbonyl Group Techniques
Carbonyl group techniques have been widely utilized in organic synthesis and industrial processes for decades. However, as the demand for more efficient and sustainable chemical processes grows, several challenges have emerged in the field. One of the primary obstacles is the need for improved selectivity in carbonyl group reactions. Many current methods struggle to achieve high levels of chemo-, regio-, and stereoselectivity, leading to unwanted side products and reduced yields.
Another significant challenge lies in the development of milder reaction conditions. Traditional carbonyl group transformations often require harsh reagents, high temperatures, or strong acids/bases, which can limit their applicability and sustainability. Researchers are actively seeking ways to perform these reactions under more environmentally friendly conditions, such as using water as a solvent or employing catalysts that operate at room temperature.
The efficiency of carbonyl group techniques is also hindered by the need for stoichiometric amounts of reagents in many cases. This not only increases the cost and environmental impact of the processes but also complicates purification steps. Developing catalytic methods that can achieve the same transformations with substoichiometric amounts of reagents remains a key focus area for improvement.
Furthermore, the scope of substrates that can be effectively transformed using current carbonyl group techniques is often limited. Many methods work well for simple model compounds but fail when applied to more complex molecules or those containing sensitive functional groups. Expanding the substrate scope while maintaining high efficiency is crucial for the broader application of these techniques in areas such as natural product synthesis and drug discovery.
The challenge of scalability also persists in carbonyl group chemistry. Reactions that perform well on a laboratory scale may encounter significant issues when scaled up for industrial production. Factors such as heat transfer, mixing efficiency, and reagent distribution can dramatically affect the outcome of carbonyl group transformations at larger scales. Developing robust and scalable processes that maintain high efficiency across different scales is essential for the practical implementation of these techniques.
Lastly, the integration of carbonyl group techniques with emerging technologies presents both opportunities and challenges. For instance, the application of flow chemistry to carbonyl group reactions shows promise for improved efficiency and control, but requires overcoming issues related to reagent solubility, reaction kinetics, and in-line purification. Similarly, the use of artificial intelligence and machine learning for reaction optimization and prediction is an exciting frontier, but requires extensive data collection and model development specific to carbonyl group chemistry.
Another significant challenge lies in the development of milder reaction conditions. Traditional carbonyl group transformations often require harsh reagents, high temperatures, or strong acids/bases, which can limit their applicability and sustainability. Researchers are actively seeking ways to perform these reactions under more environmentally friendly conditions, such as using water as a solvent or employing catalysts that operate at room temperature.
The efficiency of carbonyl group techniques is also hindered by the need for stoichiometric amounts of reagents in many cases. This not only increases the cost and environmental impact of the processes but also complicates purification steps. Developing catalytic methods that can achieve the same transformations with substoichiometric amounts of reagents remains a key focus area for improvement.
Furthermore, the scope of substrates that can be effectively transformed using current carbonyl group techniques is often limited. Many methods work well for simple model compounds but fail when applied to more complex molecules or those containing sensitive functional groups. Expanding the substrate scope while maintaining high efficiency is crucial for the broader application of these techniques in areas such as natural product synthesis and drug discovery.
The challenge of scalability also persists in carbonyl group chemistry. Reactions that perform well on a laboratory scale may encounter significant issues when scaled up for industrial production. Factors such as heat transfer, mixing efficiency, and reagent distribution can dramatically affect the outcome of carbonyl group transformations at larger scales. Developing robust and scalable processes that maintain high efficiency across different scales is essential for the practical implementation of these techniques.
Lastly, the integration of carbonyl group techniques with emerging technologies presents both opportunities and challenges. For instance, the application of flow chemistry to carbonyl group reactions shows promise for improved efficiency and control, but requires overcoming issues related to reagent solubility, reaction kinetics, and in-line purification. Similarly, the use of artificial intelligence and machine learning for reaction optimization and prediction is an exciting frontier, but requires extensive data collection and model development specific to carbonyl group chemistry.
State-of-the-Art Carbonyl Group Optimization Approaches
01 Carbonyl group reduction techniques
Various methods for reducing carbonyl groups to improve efficiency in chemical processes. These techniques involve the use of catalysts, hydrogen donors, or electrochemical methods to convert carbonyl compounds into alcohols or other reduced forms. The efficiency of these processes is crucial for industrial applications and the synthesis of complex molecules.- Carbonyl group reduction techniques: Various methods for reducing carbonyl groups to improve efficiency in organic synthesis. These techniques involve the use of catalysts, reducing agents, or electrochemical processes to convert carbonyl compounds into alcohols or other reduced forms. The efficiency of these methods is crucial for industrial applications and the production of pharmaceuticals and fine chemicals.
- Catalytic oxidation of carbonyl compounds: Efficient catalytic systems for the oxidation of carbonyl compounds, particularly aldehydes and ketones. These processes aim to improve selectivity and yield in the production of carboxylic acids or other oxidized products. The development of novel catalysts and reaction conditions enhances the overall efficiency of carbonyl group oxidation reactions.
- Carbonyl group protection strategies: Methods for protecting carbonyl groups during multi-step syntheses to prevent unwanted side reactions. These techniques involve the use of various protecting groups and their efficient installation and removal. The development of new protecting group strategies aims to improve overall synthetic efficiency and yield in complex molecule synthesis.
- Carbonyl group functionalization: Efficient methods for functionalizing carbonyl groups to introduce new chemical moieties. These techniques include aldol reactions, Mannich reactions, and other carbon-carbon bond-forming processes. The development of new catalysts and reaction conditions aims to improve the efficiency and selectivity of these transformations.
- Green chemistry approaches for carbonyl group transformations: Environmentally friendly methods for carbonyl group transformations, focusing on the use of renewable resources, catalysts, and solvents. These approaches aim to reduce waste, improve atom economy, and enhance overall process efficiency while minimizing environmental impact in carbonyl chemistry.
02 Catalytic oxidation of carbonyl compounds
Efficient catalytic systems for the oxidation of carbonyl compounds, particularly aldehydes and ketones. These methods often employ metal catalysts or organocatalysts to facilitate the conversion of carbonyl groups into carboxylic acids or other oxidized products. The focus is on improving selectivity, yield, and reducing side reactions to enhance overall process efficiency.Expand Specific Solutions03 Carbonyl group protection strategies
Techniques for protecting carbonyl groups during multi-step syntheses to prevent unwanted side reactions. This includes the use of various protecting groups such as acetals, ketals, or silyl ethers. The efficiency of protection and deprotection steps is crucial for maintaining high yields in complex synthetic pathways.Expand Specific Solutions04 Green chemistry approaches for carbonyl transformations
Environmentally friendly methods for carbonyl group transformations, focusing on reducing waste, using renewable resources, and improving atom economy. These techniques often involve the use of bio-based catalysts, solvent-free conditions, or microwave-assisted reactions to enhance efficiency while minimizing environmental impact.Expand Specific Solutions05 Carbonyl group functionalization for pharmaceutical applications
Efficient methods for functionalizing carbonyl groups in the context of drug discovery and synthesis. These techniques focus on introducing diverse functional groups to carbonyl-containing compounds, enabling the creation of complex molecular structures with potential therapeutic properties. The emphasis is on high-yielding, selective reactions that are amenable to scale-up and industrial production.Expand Specific Solutions
Key Players in Carbonyl Research and Industry
The competitive landscape for "Exploring Carbonyl Group Techniques for Optimized Efficiency" is characterized by a mature market with significant potential for growth and innovation. The global market size for carbonyl group-related technologies is substantial, driven by applications in pharmaceuticals, materials science, and chemical manufacturing. Key players like Sumitomo Chemical, DAIKIN INDUSTRIES, and Dow Global Technologies are at the forefront of research and development in this field. These companies, along with others such as Merck Patent GmbH and Wacker Chemie AG, demonstrate varying levels of technological maturity, with some focusing on advanced applications while others are still in the early stages of exploration. The involvement of academic institutions like Niigata University and The Australian National University suggests ongoing fundamental research, indicating potential for future breakthroughs and market expansion.
Dow Global Technologies LLC
Technical Solution: Dow has focused on developing sustainable carbonyl group techniques for industrial applications. Their approach utilizes bio-based feedstocks and green chemistry principles to optimize efficiency in carbonyl transformations. Dow has pioneered the use of enzymatic catalysts for selective carbonyl reductions, which operate under mild conditions and produce fewer by-products compared to traditional methods[2]. Additionally, they have implemented continuous flow reactors for carbonyl chemistry, allowing for precise control of reaction parameters and improved product quality[4].
Strengths: Environmentally friendly processes, reduced energy consumption, and high product purity. Weaknesses: Limited substrate scope for enzymatic catalysts and potential scalability challenges.
Wacker Chemie AG
Technical Solution: Wacker Chemie has focused on developing efficient carbonyl group techniques for silicon-based chemistry. Their approach involves the use of platinum catalysts for hydrosilylation reactions of carbonyl compounds, enabling the production of organosilicon materials with improved properties[7]. Wacker has also developed novel oxidation processes for the selective formation of carbonyl groups in silicone polymers, enhancing their functionality and performance in various applications[8]. Additionally, they have implemented in-line analytics and process control systems to optimize carbonyl group reactions in real-time.
Strengths: Expertise in silicon chemistry, high-value products, and efficient process control. Weaknesses: Limited applicability outside silicon-based chemistry and potential reliance on precious metal catalysts.
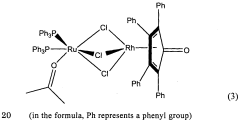
Innovative Catalysts and Reaction Mechanisms
Method for introducing carbonyl groups into polymers containing double carbon-carbon linkages
PatentWO2004104053A8
Innovation
- A selective oxygenation process using nitrous oxide to convert double carbon-carbon bonds into aldehyde and ketone groups, which can be performed with or without solvents, and involves the use of inert gases to manage reaction safety and stability, allowing for varying levels of carbonyl group introduction without high nitrogen oxide concentrations.
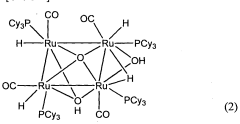
Method for producing compound with carbonyl group by using ruthenium carbonyl complex having tridentate ligand as dehydrogenation oxidation catalyst
PatentWO2012144650A1
Innovation
- A ruthenium carbonyl complex with a tridentate ligand containing two phosphino groups and a -NH- group is used as a dehydrogenation oxidation catalyst, allowing for the efficient production of carbonyl compounds like aldehydes, ketones, esters, amides, and lactones under mild conditions without toxic by-products.
Environmental Impact of Carbonyl Group Processes
The environmental impact of carbonyl group processes is a critical consideration in the pursuit of optimized efficiency. These processes, while essential in various industries, can have significant implications for ecosystems and human health if not properly managed.
One of the primary environmental concerns associated with carbonyl group processes is the emission of volatile organic compounds (VOCs). Many carbonyl compounds are highly volatile and can easily escape into the atmosphere during production, storage, or use. These emissions contribute to the formation of ground-level ozone and smog, which can have detrimental effects on air quality and respiratory health.
Water pollution is another potential consequence of carbonyl group processes. Effluents from industrial operations may contain residual carbonyl compounds or their byproducts, which can contaminate water sources if not adequately treated. This contamination can disrupt aquatic ecosystems and pose risks to human health through the consumption of contaminated water or seafood.
The production and disposal of carbonyl-containing materials also raise environmental concerns. Many of these compounds are derived from fossil fuels, contributing to carbon emissions and resource depletion. Additionally, the improper disposal of carbonyl-containing waste can lead to soil contamination and long-term environmental degradation.
However, advancements in green chemistry and sustainable manufacturing practices are helping to mitigate these environmental impacts. The development of bio-based carbonyl compounds, for instance, offers a more sustainable alternative to petroleum-derived products. These bio-based materials can reduce carbon footprints and minimize reliance on non-renewable resources.
Process optimization techniques are also playing a crucial role in reducing the environmental impact of carbonyl group processes. Improved reaction efficiency and selectivity can minimize waste generation and energy consumption. Closed-loop systems and solvent recycling technologies are being implemented to reduce emissions and conserve resources.
Furthermore, the adoption of advanced pollution control technologies is helping to minimize the release of harmful compounds into the environment. Catalytic oxidizers, scrubbers, and biofilters are being employed to capture and treat emissions from carbonyl group processes, significantly reducing their impact on air quality.
As regulations become more stringent and public awareness of environmental issues grows, industries are increasingly focusing on developing cleaner and more sustainable carbonyl group processes. This shift towards environmentally friendly practices not only benefits ecosystems and public health but also drives innovation and competitiveness in the global market.
One of the primary environmental concerns associated with carbonyl group processes is the emission of volatile organic compounds (VOCs). Many carbonyl compounds are highly volatile and can easily escape into the atmosphere during production, storage, or use. These emissions contribute to the formation of ground-level ozone and smog, which can have detrimental effects on air quality and respiratory health.
Water pollution is another potential consequence of carbonyl group processes. Effluents from industrial operations may contain residual carbonyl compounds or their byproducts, which can contaminate water sources if not adequately treated. This contamination can disrupt aquatic ecosystems and pose risks to human health through the consumption of contaminated water or seafood.
The production and disposal of carbonyl-containing materials also raise environmental concerns. Many of these compounds are derived from fossil fuels, contributing to carbon emissions and resource depletion. Additionally, the improper disposal of carbonyl-containing waste can lead to soil contamination and long-term environmental degradation.
However, advancements in green chemistry and sustainable manufacturing practices are helping to mitigate these environmental impacts. The development of bio-based carbonyl compounds, for instance, offers a more sustainable alternative to petroleum-derived products. These bio-based materials can reduce carbon footprints and minimize reliance on non-renewable resources.
Process optimization techniques are also playing a crucial role in reducing the environmental impact of carbonyl group processes. Improved reaction efficiency and selectivity can minimize waste generation and energy consumption. Closed-loop systems and solvent recycling technologies are being implemented to reduce emissions and conserve resources.
Furthermore, the adoption of advanced pollution control technologies is helping to minimize the release of harmful compounds into the environment. Catalytic oxidizers, scrubbers, and biofilters are being employed to capture and treat emissions from carbonyl group processes, significantly reducing their impact on air quality.
As regulations become more stringent and public awareness of environmental issues grows, industries are increasingly focusing on developing cleaner and more sustainable carbonyl group processes. This shift towards environmentally friendly practices not only benefits ecosystems and public health but also drives innovation and competitiveness in the global market.
Regulatory Framework for Carbonyl-Based Products
The regulatory framework for carbonyl-based products is a complex and evolving landscape that significantly impacts the development, production, and distribution of these compounds. Governments and international organizations have established comprehensive guidelines to ensure the safe handling, use, and disposal of carbonyl-containing substances, recognizing their potential environmental and health impacts.
At the forefront of these regulations are the safety data sheet (SDS) requirements, which mandate detailed information on the properties, hazards, and proper handling procedures for carbonyl compounds. These SDSs must be regularly updated to reflect the latest scientific understanding and risk assessments. Additionally, strict labeling and packaging regulations are in place to communicate potential dangers and necessary precautions to users and handlers.
Environmental protection agencies worldwide have implemented stringent emission control standards for industries utilizing carbonyl compounds. These regulations often require the installation of advanced air pollution control technologies and the implementation of best management practices to minimize the release of volatile organic compounds (VOCs) into the atmosphere.
In the pharmaceutical and food industries, regulatory bodies such as the FDA and EMA have established specific guidelines for the use of carbonyl-containing substances in products intended for human consumption. These guidelines often include limits on residual solvent levels and impurity profiles, necessitating rigorous quality control measures throughout the production process.
Occupational health and safety regulations play a crucial role in protecting workers who handle carbonyl compounds. These regulations typically mandate the use of personal protective equipment, implementation of engineering controls, and regular monitoring of workplace air quality. Furthermore, they often require comprehensive employee training programs on the safe handling and emergency response procedures for carbonyl-based materials.
The transportation of carbonyl compounds is subject to strict international regulations, such as those outlined in the UN Recommendations on the Transport of Dangerous Goods. These guidelines specify packaging requirements, labeling standards, and documentation procedures to ensure safe transport and minimize the risk of accidents or spills.
As the understanding of the environmental and health impacts of carbonyl compounds continues to evolve, regulatory frameworks are regularly updated to reflect new scientific findings. This dynamic regulatory landscape necessitates ongoing compliance efforts and adaptability from industries working with carbonyl-based products, driving innovation in safer and more sustainable practices.
At the forefront of these regulations are the safety data sheet (SDS) requirements, which mandate detailed information on the properties, hazards, and proper handling procedures for carbonyl compounds. These SDSs must be regularly updated to reflect the latest scientific understanding and risk assessments. Additionally, strict labeling and packaging regulations are in place to communicate potential dangers and necessary precautions to users and handlers.
Environmental protection agencies worldwide have implemented stringent emission control standards for industries utilizing carbonyl compounds. These regulations often require the installation of advanced air pollution control technologies and the implementation of best management practices to minimize the release of volatile organic compounds (VOCs) into the atmosphere.
In the pharmaceutical and food industries, regulatory bodies such as the FDA and EMA have established specific guidelines for the use of carbonyl-containing substances in products intended for human consumption. These guidelines often include limits on residual solvent levels and impurity profiles, necessitating rigorous quality control measures throughout the production process.
Occupational health and safety regulations play a crucial role in protecting workers who handle carbonyl compounds. These regulations typically mandate the use of personal protective equipment, implementation of engineering controls, and regular monitoring of workplace air quality. Furthermore, they often require comprehensive employee training programs on the safe handling and emergency response procedures for carbonyl-based materials.
The transportation of carbonyl compounds is subject to strict international regulations, such as those outlined in the UN Recommendations on the Transport of Dangerous Goods. These guidelines specify packaging requirements, labeling standards, and documentation procedures to ensure safe transport and minimize the risk of accidents or spills.
As the understanding of the environmental and health impacts of carbonyl compounds continues to evolve, regulatory frameworks are regularly updated to reflect new scientific findings. This dynamic regulatory landscape necessitates ongoing compliance efforts and adaptability from industries working with carbonyl-based products, driving innovation in safer and more sustainable practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!