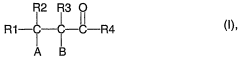
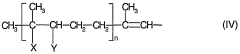
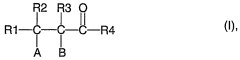
How to Address Challenges in Carbonyl Compound Synthesis?
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Synthesis Background and Objectives
Carbonyl compounds play a crucial role in organic synthesis, serving as versatile building blocks for a wide range of chemical transformations. The synthesis of these compounds has been a cornerstone of organic chemistry for over a century, with continuous advancements in methodologies and applications. The primary objective of this technical research report is to address the challenges in carbonyl compound synthesis and explore innovative solutions to overcome existing limitations.
The evolution of carbonyl synthesis techniques has been marked by significant milestones, from classical methods like Friedel-Crafts acylation to modern catalytic approaches. Early developments focused on using strong oxidizing agents and harsh reaction conditions, which often led to poor selectivity and limited functional group tolerance. As the field progressed, chemists sought more efficient and environmentally friendly methods, leading to the development of transition metal-catalyzed carbonylation reactions and organocatalytic processes.
Current trends in carbonyl synthesis are driven by the need for sustainable and atom-economical processes. Green chemistry principles have become increasingly important, pushing researchers to develop methods that minimize waste generation and utilize renewable resources. Additionally, the pharmaceutical and fine chemical industries demand highly selective and scalable carbonyl synthesis methods, particularly for the production of complex molecules and natural product analogues.
One of the primary challenges in carbonyl compound synthesis is the control of chemoselectivity and regioselectivity. Many traditional methods suffer from poor selectivity, leading to mixtures of products and reduced yields. Another significant hurdle is the development of mild and functional group-tolerant conditions, which are essential for late-stage functionalization in complex molecule synthesis. Furthermore, the use of toxic or expensive reagents and catalysts remains a concern in many carbonyl synthesis protocols.
To address these challenges, researchers are exploring various innovative approaches. One promising direction is the development of new catalytic systems, including transition metal complexes and organocatalysts, which can promote carbonyl formation under milder conditions and with improved selectivity. Another area of focus is the utilization of renewable feedstocks and bio-based starting materials for carbonyl synthesis, aligning with the principles of sustainable chemistry.
The integration of enabling technologies, such as flow chemistry and photocatalysis, is also expected to revolutionize carbonyl compound synthesis. These technologies offer improved control over reaction parameters, enhanced safety profiles, and the potential for continuous production. Additionally, computational methods and artificial intelligence are increasingly being employed to predict reaction outcomes and optimize synthetic routes, potentially accelerating the discovery of novel carbonyl synthesis methodologies.
The evolution of carbonyl synthesis techniques has been marked by significant milestones, from classical methods like Friedel-Crafts acylation to modern catalytic approaches. Early developments focused on using strong oxidizing agents and harsh reaction conditions, which often led to poor selectivity and limited functional group tolerance. As the field progressed, chemists sought more efficient and environmentally friendly methods, leading to the development of transition metal-catalyzed carbonylation reactions and organocatalytic processes.
Current trends in carbonyl synthesis are driven by the need for sustainable and atom-economical processes. Green chemistry principles have become increasingly important, pushing researchers to develop methods that minimize waste generation and utilize renewable resources. Additionally, the pharmaceutical and fine chemical industries demand highly selective and scalable carbonyl synthesis methods, particularly for the production of complex molecules and natural product analogues.
One of the primary challenges in carbonyl compound synthesis is the control of chemoselectivity and regioselectivity. Many traditional methods suffer from poor selectivity, leading to mixtures of products and reduced yields. Another significant hurdle is the development of mild and functional group-tolerant conditions, which are essential for late-stage functionalization in complex molecule synthesis. Furthermore, the use of toxic or expensive reagents and catalysts remains a concern in many carbonyl synthesis protocols.
To address these challenges, researchers are exploring various innovative approaches. One promising direction is the development of new catalytic systems, including transition metal complexes and organocatalysts, which can promote carbonyl formation under milder conditions and with improved selectivity. Another area of focus is the utilization of renewable feedstocks and bio-based starting materials for carbonyl synthesis, aligning with the principles of sustainable chemistry.
The integration of enabling technologies, such as flow chemistry and photocatalysis, is also expected to revolutionize carbonyl compound synthesis. These technologies offer improved control over reaction parameters, enhanced safety profiles, and the potential for continuous production. Additionally, computational methods and artificial intelligence are increasingly being employed to predict reaction outcomes and optimize synthetic routes, potentially accelerating the discovery of novel carbonyl synthesis methodologies.
Market Demand Analysis for Carbonyl Compounds
The market demand for carbonyl compounds has been experiencing significant growth, driven by their widespread applications across various industries. These versatile organic compounds play crucial roles in pharmaceuticals, agrochemicals, fragrances, and materials science, making them indispensable in modern industrial processes.
In the pharmaceutical sector, carbonyl compounds serve as essential building blocks for drug synthesis. The increasing global demand for novel therapeutics, coupled with the rising prevalence of chronic diseases, has led to a surge in pharmaceutical research and development activities. This trend directly translates to a growing need for carbonyl compounds in drug discovery and manufacturing processes.
The agrochemical industry also heavily relies on carbonyl compounds for the production of pesticides, herbicides, and plant growth regulators. As the global population continues to expand, the pressure on agricultural productivity intensifies, driving the demand for more effective and environmentally friendly agrochemicals. This, in turn, fuels the market for carbonyl compounds used in their synthesis.
In the fragrance and flavor industry, carbonyl compounds are key ingredients in creating a wide range of scents and tastes. The growing consumer preference for natural and organic products has led to increased research into bio-based carbonyl compounds, opening new avenues for market growth. Additionally, the expanding personal care and cosmetics industry contributes to the rising demand for carbonyl compounds used in perfumes and other scented products.
The materials science sector utilizes carbonyl compounds in the production of polymers, resins, and advanced materials. With the ongoing technological advancements in industries such as automotive, aerospace, and electronics, the demand for high-performance materials continues to grow, subsequently driving the market for carbonyl compounds.
Geographically, Asia-Pacific has emerged as a major consumer and producer of carbonyl compounds, primarily due to the rapid industrialization in countries like China and India. North America and Europe maintain significant market shares, driven by their well-established pharmaceutical and chemical industries.
The market for carbonyl compounds is expected to continue its upward trajectory in the coming years. However, challenges such as stringent environmental regulations and the need for more sustainable production methods are shaping the industry landscape. This has led to increased focus on developing green chemistry approaches for carbonyl compound synthesis, presenting both challenges and opportunities for market players.
As the demand for carbonyl compounds grows, addressing the challenges in their synthesis becomes increasingly critical. Innovations in catalysis, flow chemistry, and biocatalysis are expected to play pivotal roles in overcoming current limitations and meeting the evolving market needs. The industry's ability to develop more efficient, cost-effective, and environmentally friendly synthesis methods will be key to sustaining growth and meeting the diverse demands across multiple sectors.
In the pharmaceutical sector, carbonyl compounds serve as essential building blocks for drug synthesis. The increasing global demand for novel therapeutics, coupled with the rising prevalence of chronic diseases, has led to a surge in pharmaceutical research and development activities. This trend directly translates to a growing need for carbonyl compounds in drug discovery and manufacturing processes.
The agrochemical industry also heavily relies on carbonyl compounds for the production of pesticides, herbicides, and plant growth regulators. As the global population continues to expand, the pressure on agricultural productivity intensifies, driving the demand for more effective and environmentally friendly agrochemicals. This, in turn, fuels the market for carbonyl compounds used in their synthesis.
In the fragrance and flavor industry, carbonyl compounds are key ingredients in creating a wide range of scents and tastes. The growing consumer preference for natural and organic products has led to increased research into bio-based carbonyl compounds, opening new avenues for market growth. Additionally, the expanding personal care and cosmetics industry contributes to the rising demand for carbonyl compounds used in perfumes and other scented products.
The materials science sector utilizes carbonyl compounds in the production of polymers, resins, and advanced materials. With the ongoing technological advancements in industries such as automotive, aerospace, and electronics, the demand for high-performance materials continues to grow, subsequently driving the market for carbonyl compounds.
Geographically, Asia-Pacific has emerged as a major consumer and producer of carbonyl compounds, primarily due to the rapid industrialization in countries like China and India. North America and Europe maintain significant market shares, driven by their well-established pharmaceutical and chemical industries.
The market for carbonyl compounds is expected to continue its upward trajectory in the coming years. However, challenges such as stringent environmental regulations and the need for more sustainable production methods are shaping the industry landscape. This has led to increased focus on developing green chemistry approaches for carbonyl compound synthesis, presenting both challenges and opportunities for market players.
As the demand for carbonyl compounds grows, addressing the challenges in their synthesis becomes increasingly critical. Innovations in catalysis, flow chemistry, and biocatalysis are expected to play pivotal roles in overcoming current limitations and meeting the evolving market needs. The industry's ability to develop more efficient, cost-effective, and environmentally friendly synthesis methods will be key to sustaining growth and meeting the diverse demands across multiple sectors.
Current Challenges in Carbonyl Compound Synthesis
Carbonyl compound synthesis remains a cornerstone of organic chemistry, yet it continues to face significant challenges that hinder its efficiency and sustainability. One of the primary obstacles is the reliance on toxic and environmentally harmful reagents. Many traditional methods for carbonyl synthesis involve the use of heavy metals, strong oxidizing agents, or corrosive substances, which pose risks to both human health and the environment.
Another major challenge lies in achieving high selectivity, particularly in complex molecular structures. Controlling the regioselectivity and stereoselectivity of carbonyl formation can be extremely difficult, especially when dealing with multifunctional substrates. This often results in the need for additional protection and deprotection steps, reducing overall synthetic efficiency and atom economy.
The energy intensity of many carbonyl synthesis processes presents a further hurdle. Numerous reactions require elevated temperatures or prolonged reaction times, leading to high energy consumption and increased production costs. This energy demand not only impacts the economic viability of large-scale syntheses but also contributes to the carbon footprint of chemical manufacturing.
Scalability remains a persistent issue in carbonyl compound synthesis. Methods that work well on a laboratory scale often face significant challenges when scaled up for industrial production. Issues such as heat transfer, mixing efficiency, and product isolation can become problematic at larger scales, necessitating extensive process optimization or alternative synthetic routes.
The limited substrate scope of many carbonyl synthesis methods also poses a challenge. Certain reactions may work well for simple molecules but fail when applied to more complex or functionalized substrates. This limitation can force chemists to employ longer, less efficient synthetic routes or to rely on protecting group strategies, further complicating the synthesis process.
Sustainability concerns have brought attention to the need for greener approaches in carbonyl synthesis. The use of renewable feedstocks, bio-based catalysts, and more environmentally benign solvents is becoming increasingly important. However, developing sustainable methods that can match or exceed the efficiency and versatility of traditional approaches remains a significant challenge.
Lastly, the development of novel, more efficient catalysts for carbonyl synthesis continues to be an area of intense research. While significant progress has been made in homogeneous and heterogeneous catalysis, there is still a need for catalysts that can operate under milder conditions, with broader substrate scopes, and with improved selectivity. The design of such catalysts often requires a delicate balance between activity, selectivity, and stability, presenting a complex challenge for synthetic chemists and materials scientists alike.
Another major challenge lies in achieving high selectivity, particularly in complex molecular structures. Controlling the regioselectivity and stereoselectivity of carbonyl formation can be extremely difficult, especially when dealing with multifunctional substrates. This often results in the need for additional protection and deprotection steps, reducing overall synthetic efficiency and atom economy.
The energy intensity of many carbonyl synthesis processes presents a further hurdle. Numerous reactions require elevated temperatures or prolonged reaction times, leading to high energy consumption and increased production costs. This energy demand not only impacts the economic viability of large-scale syntheses but also contributes to the carbon footprint of chemical manufacturing.
Scalability remains a persistent issue in carbonyl compound synthesis. Methods that work well on a laboratory scale often face significant challenges when scaled up for industrial production. Issues such as heat transfer, mixing efficiency, and product isolation can become problematic at larger scales, necessitating extensive process optimization or alternative synthetic routes.
The limited substrate scope of many carbonyl synthesis methods also poses a challenge. Certain reactions may work well for simple molecules but fail when applied to more complex or functionalized substrates. This limitation can force chemists to employ longer, less efficient synthetic routes or to rely on protecting group strategies, further complicating the synthesis process.
Sustainability concerns have brought attention to the need for greener approaches in carbonyl synthesis. The use of renewable feedstocks, bio-based catalysts, and more environmentally benign solvents is becoming increasingly important. However, developing sustainable methods that can match or exceed the efficiency and versatility of traditional approaches remains a significant challenge.
Lastly, the development of novel, more efficient catalysts for carbonyl synthesis continues to be an area of intense research. While significant progress has been made in homogeneous and heterogeneous catalysis, there is still a need for catalysts that can operate under milder conditions, with broader substrate scopes, and with improved selectivity. The design of such catalysts often requires a delicate balance between activity, selectivity, and stability, presenting a complex challenge for synthetic chemists and materials scientists alike.
Existing Carbonyl Synthesis Strategies
01 Synthesis of carbonyl compounds
Various methods for synthesizing carbonyl compounds are described, including oxidation reactions, rearrangements, and catalytic processes. These techniques allow for the production of a wide range of aldehydes and ketones with different functional groups and structural features.- Synthesis of carbonyl compounds: Various methods for synthesizing carbonyl compounds are described, including oxidation reactions, rearrangements, and catalytic processes. These techniques allow for the production of a wide range of aldehydes and ketones with different functional groups and structural features.
- Reactions and transformations of carbonyl compounds: Carbonyl compounds undergo numerous reactions and transformations, such as condensations, reductions, and additions. These processes are utilized in the synthesis of complex organic molecules and the production of industrially important chemicals.
- Analysis and detection of carbonyl compounds: Methods for analyzing and detecting carbonyl compounds in various matrices are presented. These techniques include spectroscopic methods, chromatographic separations, and chemical derivatization approaches, enabling accurate quantification and identification of carbonyl species.
- Applications of carbonyl compounds in industry: Carbonyl compounds find extensive applications in various industries, including pharmaceuticals, polymers, and fine chemicals. They serve as key intermediates and building blocks in the synthesis of complex molecules and materials with diverse properties and functions.
- Carbonyl compounds in biological systems: The role of carbonyl compounds in biological systems is explored, including their involvement in metabolic processes, cellular signaling, and potential health effects. Research focuses on understanding the formation, reactivity, and impact of these compounds in living organisms.
02 Detection and analysis of carbonyl compounds
Techniques for detecting and analyzing carbonyl compounds in various samples are presented. These methods may involve spectroscopic analysis, chemical derivatization, or specialized reagents to identify and quantify carbonyl-containing molecules in complex mixtures.Expand Specific Solutions03 Reactions and transformations of carbonyl compounds
Carbonyl compounds undergo various reactions and transformations, including condensation, reduction, and addition reactions. These processes are essential in organic synthesis and can be used to create more complex molecules or modify existing compounds.Expand Specific Solutions04 Applications of carbonyl compounds in industry
Carbonyl compounds find numerous applications in various industries, including pharmaceuticals, fragrances, and materials science. They serve as important intermediates in the production of drugs, polymers, and other valuable products.Expand Specific Solutions05 Environmental and health considerations of carbonyl compounds
The environmental impact and health effects of carbonyl compounds are discussed, including their role in atmospheric chemistry and potential toxicity. Methods for mitigating exposure and reducing emissions of these compounds are also explored.Expand Specific Solutions
Key Players in Carbonyl Compound Industry
The carbonyl compound synthesis market is in a mature stage, with established players and well-defined processes. However, ongoing challenges in efficiency, selectivity, and sustainability drive continued innovation. The global market size for carbonyl compounds is estimated to be in the billions of dollars, with steady growth projected. Technologically, the field is advanced but evolving, as companies like Sumitomo Chemical, BASF, and Umicore lead efforts to develop greener synthesis methods and novel catalysts. Academic institutions such as the University of Zurich and University of Houston collaborate with industry to address fundamental challenges. Emerging players like Saltigo GmbH and Resonac Holdings are also making significant contributions, particularly in custom synthesis and specialty chemicals.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed innovative approaches to carbonyl compound synthesis, focusing on green chemistry principles and atom-efficient processes. They have made significant progress in electrochemical methods for carbonyl formation, utilizing renewable electricity to drive oxidations and reductions [13]. Their research includes the development of novel electrode materials and electrolyte systems for selective carbonyl synthesis. Sumitomo has also explored the use of supercritical CO2 as a solvent and reagent in carbonyl-forming reactions, offering environmentally benign alternatives to traditional organic solvents [15].
Strengths: Leadership in electrochemical synthesis, focus on green solvents and reagents, and expertise in process intensification. Weaknesses: Potential high energy costs for electrochemical processes, scalability challenges for some novel methods.
BASF Corp.
Technical Solution: BASF has developed innovative catalytic processes for carbonyl compound synthesis, focusing on sustainable and efficient methods. Their approach includes the use of metal-organic frameworks (MOFs) as heterogeneous catalysts for aldol condensations and oxidations [1]. They have also implemented continuous flow chemistry techniques for the production of aldehydes and ketones, which allows for better control of reaction parameters and improved yields [3]. BASF's research extends to biocatalytic methods, utilizing engineered enzymes for stereoselective carbonyl compound synthesis, particularly in pharmaceutical intermediates [5].
Strengths: Diverse portfolio of catalytic methods, integration of green chemistry principles, and scalable industrial processes. Weaknesses: High research and development costs, potential dependence on precious metal catalysts.
Innovative Approaches in Carbonyl Formation
Continuous method for the production of carbonyl compounds
PatentWO2004022515A1
Innovation
- A continuous process involving the simultaneous reaction and separation of carbonyl compounds in a single reactor using countercurrent flow and reactive distillation, where the carbonyl compounds are fed continuously over a catalyst bed in a reaction column, allowing for high selectivities and efficient removal of reaction products, thereby minimizing excess reactant usage and energy consumption.
Patent
Innovation
- Novel catalytic systems for selective carbonyl compound synthesis, reducing side reactions and improving yield.
- Development of milder reaction conditions to expand substrate scope and functional group tolerance in carbonyl synthesis.
- Implementation of chemoselective methods for carbonyl formation in complex molecules with multiple functional groups.
Green Chemistry in Carbonyl Synthesis
Green chemistry principles are increasingly being applied to carbonyl compound synthesis to address environmental and sustainability challenges. This approach focuses on developing more efficient, less toxic, and environmentally friendly methods for producing carbonyl compounds, which are essential building blocks in organic synthesis.
One of the key strategies in green carbonyl synthesis is the use of alternative solvents. Traditional organic solvents are often toxic, flammable, and contribute to air pollution. Water, as a green solvent, has gained significant attention due to its abundance, non-toxicity, and low cost. Researchers have developed water-compatible catalysts and reaction conditions that allow for efficient carbonyl synthesis in aqueous media. Additionally, ionic liquids and supercritical fluids are being explored as environmentally benign alternatives to conventional organic solvents.
Catalysis plays a crucial role in green carbonyl synthesis. Heterogeneous catalysts, which can be easily separated and recycled, are preferred over homogeneous catalysts. Metal nanoparticles supported on various materials have shown promising results in catalyzing carbonyl formation reactions. Biocatalysts, such as enzymes, offer another green alternative, providing high selectivity and operating under mild conditions.
Energy efficiency is another important aspect of green chemistry in carbonyl synthesis. Microwave-assisted reactions have gained popularity due to their ability to accelerate reactions and reduce energy consumption. Photochemical methods, utilizing visible light as an energy source, are also being explored for carbonyl compound synthesis, offering a clean and renewable approach.
Atom economy is a fundamental principle of green chemistry that is being applied to carbonyl synthesis. Researchers are developing new synthetic routes that maximize the incorporation of reactants into the final product, minimizing waste generation. This includes the use of multicomponent reactions and cascade processes that combine multiple steps into a single operation.
Renewable feedstocks are increasingly being used as starting materials for carbonyl compound synthesis. Biomass-derived platform chemicals, such as furfural and 5-hydroxymethylfurfural, are being explored as sustainable alternatives to petroleum-based precursors. These bio-based starting materials not only reduce reliance on fossil resources but also contribute to the development of a circular economy.
In conclusion, green chemistry approaches to carbonyl compound synthesis are addressing many of the challenges associated with traditional methods. By focusing on environmentally friendly solvents, efficient catalysts, energy-saving techniques, atom economy, and renewable resources, researchers are developing more sustainable processes for producing these important chemical building blocks.
One of the key strategies in green carbonyl synthesis is the use of alternative solvents. Traditional organic solvents are often toxic, flammable, and contribute to air pollution. Water, as a green solvent, has gained significant attention due to its abundance, non-toxicity, and low cost. Researchers have developed water-compatible catalysts and reaction conditions that allow for efficient carbonyl synthesis in aqueous media. Additionally, ionic liquids and supercritical fluids are being explored as environmentally benign alternatives to conventional organic solvents.
Catalysis plays a crucial role in green carbonyl synthesis. Heterogeneous catalysts, which can be easily separated and recycled, are preferred over homogeneous catalysts. Metal nanoparticles supported on various materials have shown promising results in catalyzing carbonyl formation reactions. Biocatalysts, such as enzymes, offer another green alternative, providing high selectivity and operating under mild conditions.
Energy efficiency is another important aspect of green chemistry in carbonyl synthesis. Microwave-assisted reactions have gained popularity due to their ability to accelerate reactions and reduce energy consumption. Photochemical methods, utilizing visible light as an energy source, are also being explored for carbonyl compound synthesis, offering a clean and renewable approach.
Atom economy is a fundamental principle of green chemistry that is being applied to carbonyl synthesis. Researchers are developing new synthetic routes that maximize the incorporation of reactants into the final product, minimizing waste generation. This includes the use of multicomponent reactions and cascade processes that combine multiple steps into a single operation.
Renewable feedstocks are increasingly being used as starting materials for carbonyl compound synthesis. Biomass-derived platform chemicals, such as furfural and 5-hydroxymethylfurfural, are being explored as sustainable alternatives to petroleum-based precursors. These bio-based starting materials not only reduce reliance on fossil resources but also contribute to the development of a circular economy.
In conclusion, green chemistry approaches to carbonyl compound synthesis are addressing many of the challenges associated with traditional methods. By focusing on environmentally friendly solvents, efficient catalysts, energy-saving techniques, atom economy, and renewable resources, researchers are developing more sustainable processes for producing these important chemical building blocks.
Scalability and Industrial Applications
The scalability and industrial applications of carbonyl compound synthesis present both challenges and opportunities for the chemical industry. As production scales increase, maintaining reaction efficiency and product quality becomes increasingly complex. One key challenge is heat management in large-scale reactors, particularly for exothermic carbonyl-forming reactions. Advanced reactor designs incorporating improved heat exchange systems and precise temperature control mechanisms are being developed to address this issue.
Another critical aspect of scalability is the optimization of reaction conditions for industrial-scale production. This includes fine-tuning parameters such as reagent concentrations, reaction times, and catalyst loadings to maximize yield and minimize waste. Continuous flow chemistry has emerged as a promising approach for scaling up carbonyl compound synthesis, offering benefits such as improved heat transfer, enhanced mixing, and easier process control.
The industrial applications of carbonyl compounds are vast and diverse, spanning sectors such as pharmaceuticals, agrochemicals, and materials science. In the pharmaceutical industry, carbonyl compounds serve as crucial intermediates in the synthesis of many active pharmaceutical ingredients (APIs). The ability to scale up carbonyl compound production efficiently is essential for meeting the growing demand for medications worldwide.
In the agrochemical sector, carbonyl compounds play a vital role in the production of pesticides, herbicides, and plant growth regulators. The scalability of synthesis processes directly impacts the availability and cost-effectiveness of these products, which are critical for global food security. Additionally, the materials science industry relies on carbonyl compounds for the production of polymers, resins, and advanced materials with specific properties.
Environmental considerations are becoming increasingly important in industrial-scale carbonyl compound synthesis. Green chemistry principles are being applied to develop more sustainable processes, including the use of renewable feedstocks, catalysts that enable milder reaction conditions, and solvent-free or aqueous reaction systems. These approaches not only address environmental concerns but also often lead to more economically viable processes at scale.
The integration of automation and digital technologies is revolutionizing the scalability of carbonyl compound synthesis. Advanced process analytical technologies (PAT) enable real-time monitoring and control of reactions, ensuring consistent product quality across large-scale production runs. Machine learning algorithms are being employed to optimize reaction conditions and predict scalability challenges, significantly reducing the time and resources required for process development.
Another critical aspect of scalability is the optimization of reaction conditions for industrial-scale production. This includes fine-tuning parameters such as reagent concentrations, reaction times, and catalyst loadings to maximize yield and minimize waste. Continuous flow chemistry has emerged as a promising approach for scaling up carbonyl compound synthesis, offering benefits such as improved heat transfer, enhanced mixing, and easier process control.
The industrial applications of carbonyl compounds are vast and diverse, spanning sectors such as pharmaceuticals, agrochemicals, and materials science. In the pharmaceutical industry, carbonyl compounds serve as crucial intermediates in the synthesis of many active pharmaceutical ingredients (APIs). The ability to scale up carbonyl compound production efficiently is essential for meeting the growing demand for medications worldwide.
In the agrochemical sector, carbonyl compounds play a vital role in the production of pesticides, herbicides, and plant growth regulators. The scalability of synthesis processes directly impacts the availability and cost-effectiveness of these products, which are critical for global food security. Additionally, the materials science industry relies on carbonyl compounds for the production of polymers, resins, and advanced materials with specific properties.
Environmental considerations are becoming increasingly important in industrial-scale carbonyl compound synthesis. Green chemistry principles are being applied to develop more sustainable processes, including the use of renewable feedstocks, catalysts that enable milder reaction conditions, and solvent-free or aqueous reaction systems. These approaches not only address environmental concerns but also often lead to more economically viable processes at scale.
The integration of automation and digital technologies is revolutionizing the scalability of carbonyl compound synthesis. Advanced process analytical technologies (PAT) enable real-time monitoring and control of reactions, ensuring consistent product quality across large-scale production runs. Machine learning algorithms are being employed to optimize reaction conditions and predict scalability challenges, significantly reducing the time and resources required for process development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!