How to Analyze Carbonyl Structures Using NMR?
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Carbonyl Analysis Background and Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a powerful analytical tool for elucidating the structure of organic compounds, particularly those containing carbonyl groups. The analysis of carbonyl structures using NMR has a rich history dating back to the mid-20th century, with significant advancements in both instrumentation and methodologies over the decades.
The evolution of NMR technology has been marked by continuous improvements in magnetic field strength, pulse sequences, and data processing techniques. These advancements have greatly enhanced the sensitivity and resolution of NMR spectra, allowing for more precise analysis of carbonyl compounds. The development of multidimensional NMR techniques has further expanded the capabilities of carbonyl structure elucidation, enabling the determination of complex molecular architectures.
In the context of carbonyl analysis, NMR spectroscopy offers unique insights into the electronic environment of the carbonyl carbon and its neighboring atoms. The chemical shift of the carbonyl carbon, typically observed in the range of 160-220 ppm in 13C NMR spectra, provides valuable information about the nature of the carbonyl group and its surrounding chemical environment.
The primary objective of analyzing carbonyl structures using NMR is to accurately determine the structural features of carbonyl-containing compounds. This includes identifying the type of carbonyl group (e.g., aldehyde, ketone, carboxylic acid, ester), its position within the molecule, and its interactions with neighboring functional groups. Additionally, NMR analysis aims to elucidate the stereochemistry and conformational preferences of carbonyl-containing molecules.
Another crucial goal is to understand the electronic properties of carbonyl groups and their influence on molecular reactivity. NMR data can provide insights into the electron density distribution around the carbonyl carbon, which is essential for predicting and interpreting chemical reactions involving these functional groups.
Furthermore, the integration of NMR techniques with other analytical methods, such as mass spectrometry and infrared spectroscopy, has become increasingly important. This multi-pronged approach allows for a more comprehensive characterization of carbonyl structures, addressing limitations of individual techniques and providing a more robust structural analysis.
As research in this field progresses, there is a growing emphasis on developing more sensitive and selective NMR methods for carbonyl analysis. This includes the optimization of pulse sequences specifically tailored for carbonyl detection and the exploration of novel NMR-active nuclei that can offer complementary information about carbonyl environments.
The evolution of NMR technology has been marked by continuous improvements in magnetic field strength, pulse sequences, and data processing techniques. These advancements have greatly enhanced the sensitivity and resolution of NMR spectra, allowing for more precise analysis of carbonyl compounds. The development of multidimensional NMR techniques has further expanded the capabilities of carbonyl structure elucidation, enabling the determination of complex molecular architectures.
In the context of carbonyl analysis, NMR spectroscopy offers unique insights into the electronic environment of the carbonyl carbon and its neighboring atoms. The chemical shift of the carbonyl carbon, typically observed in the range of 160-220 ppm in 13C NMR spectra, provides valuable information about the nature of the carbonyl group and its surrounding chemical environment.
The primary objective of analyzing carbonyl structures using NMR is to accurately determine the structural features of carbonyl-containing compounds. This includes identifying the type of carbonyl group (e.g., aldehyde, ketone, carboxylic acid, ester), its position within the molecule, and its interactions with neighboring functional groups. Additionally, NMR analysis aims to elucidate the stereochemistry and conformational preferences of carbonyl-containing molecules.
Another crucial goal is to understand the electronic properties of carbonyl groups and their influence on molecular reactivity. NMR data can provide insights into the electron density distribution around the carbonyl carbon, which is essential for predicting and interpreting chemical reactions involving these functional groups.
Furthermore, the integration of NMR techniques with other analytical methods, such as mass spectrometry and infrared spectroscopy, has become increasingly important. This multi-pronged approach allows for a more comprehensive characterization of carbonyl structures, addressing limitations of individual techniques and providing a more robust structural analysis.
As research in this field progresses, there is a growing emphasis on developing more sensitive and selective NMR methods for carbonyl analysis. This includes the optimization of pulse sequences specifically tailored for carbonyl detection and the exploration of novel NMR-active nuclei that can offer complementary information about carbonyl environments.
Market Demand for NMR Carbonyl Structure Analysis
The market demand for NMR carbonyl structure analysis has been steadily growing, driven by the increasing need for accurate molecular characterization in various industries. Pharmaceutical and biotechnology sectors are the primary drivers of this demand, as carbonyl structures play a crucial role in drug discovery and development processes. The ability to precisely analyze these structures using NMR techniques is essential for understanding drug-target interactions, optimizing lead compounds, and ensuring the quality and safety of pharmaceutical products.
In the chemical industry, NMR carbonyl structure analysis is vital for quality control and process optimization. Manufacturers of fine chemicals, polymers, and specialty materials rely on this technique to verify product composition and purity. The growing emphasis on sustainable and green chemistry has further boosted the demand for NMR analysis, as it allows for the development of more efficient and environmentally friendly production processes.
The food and beverage industry is another significant market for NMR carbonyl structure analysis. With increasing consumer awareness and regulatory scrutiny regarding food safety and authenticity, manufacturers are turning to advanced analytical techniques to ensure product quality. NMR analysis helps in detecting adulterants, verifying ingredient origins, and monitoring flavor compounds, all of which are critical for maintaining consumer trust and compliance with food safety regulations.
Academic and research institutions contribute substantially to the market demand for NMR carbonyl structure analysis. As the complexity of molecular research increases, the need for sophisticated analytical tools grows correspondingly. NMR spectroscopy remains a cornerstone technique in organic chemistry, biochemistry, and materials science research, driving continuous innovation in NMR technology and methodologies.
The global NMR spectroscopy market, which includes carbonyl structure analysis, is projected to experience significant growth in the coming years. This growth is fueled by technological advancements in NMR instrumentation, such as higher field strengths, improved probe designs, and enhanced data processing capabilities. These innovations are making NMR analysis more accessible, efficient, and applicable to a wider range of molecular structures and sample types.
Emerging applications in fields such as metabolomics, natural products research, and structural biology are expected to further expand the market for NMR carbonyl structure analysis. As researchers delve deeper into complex biological systems and seek to understand the intricate relationships between molecular structure and function, the demand for high-resolution NMR techniques is likely to increase.
In the chemical industry, NMR carbonyl structure analysis is vital for quality control and process optimization. Manufacturers of fine chemicals, polymers, and specialty materials rely on this technique to verify product composition and purity. The growing emphasis on sustainable and green chemistry has further boosted the demand for NMR analysis, as it allows for the development of more efficient and environmentally friendly production processes.
The food and beverage industry is another significant market for NMR carbonyl structure analysis. With increasing consumer awareness and regulatory scrutiny regarding food safety and authenticity, manufacturers are turning to advanced analytical techniques to ensure product quality. NMR analysis helps in detecting adulterants, verifying ingredient origins, and monitoring flavor compounds, all of which are critical for maintaining consumer trust and compliance with food safety regulations.
Academic and research institutions contribute substantially to the market demand for NMR carbonyl structure analysis. As the complexity of molecular research increases, the need for sophisticated analytical tools grows correspondingly. NMR spectroscopy remains a cornerstone technique in organic chemistry, biochemistry, and materials science research, driving continuous innovation in NMR technology and methodologies.
The global NMR spectroscopy market, which includes carbonyl structure analysis, is projected to experience significant growth in the coming years. This growth is fueled by technological advancements in NMR instrumentation, such as higher field strengths, improved probe designs, and enhanced data processing capabilities. These innovations are making NMR analysis more accessible, efficient, and applicable to a wider range of molecular structures and sample types.
Emerging applications in fields such as metabolomics, natural products research, and structural biology are expected to further expand the market for NMR carbonyl structure analysis. As researchers delve deeper into complex biological systems and seek to understand the intricate relationships between molecular structure and function, the demand for high-resolution NMR techniques is likely to increase.
Current Challenges in NMR Carbonyl Analysis
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique for elucidating the structure of organic compounds, including those containing carbonyl groups. However, the analysis of carbonyl structures using NMR faces several challenges that researchers and analysts must overcome to obtain accurate and reliable results.
One of the primary challenges in NMR carbonyl analysis is the relatively low sensitivity of carbonyl carbon nuclei. Carbonyl carbons typically have long relaxation times and low natural abundance, resulting in weak signal intensity. This can make it difficult to detect and accurately analyze carbonyl groups, especially in complex molecules or dilute samples.
Signal overlap is another significant issue in NMR carbonyl analysis. In complex organic molecules, the chemical shifts of carbonyl carbons may overlap with other carbon signals, making it challenging to distinguish and assign individual carbonyl resonances. This problem is exacerbated in molecules containing multiple carbonyl groups or in mixtures of compounds.
The anisotropic effects of carbonyl groups can also complicate NMR analysis. The electron-withdrawing nature of the carbonyl group can create local magnetic field inhomogeneities, leading to broadening of nearby proton signals and potentially obscuring important structural information. This effect can be particularly problematic when analyzing the protons adjacent to carbonyl groups.
Another challenge is the limited information provided by standard one-dimensional NMR experiments for carbonyl analysis. While 13C NMR can detect carbonyl carbons, it often lacks the sensitivity and resolution needed for detailed structural analysis. This limitation necessitates the use of more advanced NMR techniques, which may require specialized equipment and expertise.
The dynamic nature of some carbonyl-containing compounds poses additional challenges. Tautomerism, conformational changes, and chemical exchange processes can lead to complex NMR spectra that are difficult to interpret. These dynamic processes can cause signal broadening, coalescence, or the appearance of multiple resonances for a single carbonyl group.
Sample preparation and experimental conditions also play crucial roles in NMR carbonyl analysis. Factors such as solvent choice, temperature, and pH can significantly affect the chemical shifts and coupling patterns of carbonyl groups. Optimizing these parameters for each specific sample can be time-consuming and may require extensive method development.
Lastly, the quantitative analysis of carbonyl groups using NMR remains challenging. Variations in relaxation times and nuclear Overhauser effects can lead to inaccuracies in signal integration, making it difficult to determine the relative abundance of different carbonyl species in a sample.
One of the primary challenges in NMR carbonyl analysis is the relatively low sensitivity of carbonyl carbon nuclei. Carbonyl carbons typically have long relaxation times and low natural abundance, resulting in weak signal intensity. This can make it difficult to detect and accurately analyze carbonyl groups, especially in complex molecules or dilute samples.
Signal overlap is another significant issue in NMR carbonyl analysis. In complex organic molecules, the chemical shifts of carbonyl carbons may overlap with other carbon signals, making it challenging to distinguish and assign individual carbonyl resonances. This problem is exacerbated in molecules containing multiple carbonyl groups or in mixtures of compounds.
The anisotropic effects of carbonyl groups can also complicate NMR analysis. The electron-withdrawing nature of the carbonyl group can create local magnetic field inhomogeneities, leading to broadening of nearby proton signals and potentially obscuring important structural information. This effect can be particularly problematic when analyzing the protons adjacent to carbonyl groups.
Another challenge is the limited information provided by standard one-dimensional NMR experiments for carbonyl analysis. While 13C NMR can detect carbonyl carbons, it often lacks the sensitivity and resolution needed for detailed structural analysis. This limitation necessitates the use of more advanced NMR techniques, which may require specialized equipment and expertise.
The dynamic nature of some carbonyl-containing compounds poses additional challenges. Tautomerism, conformational changes, and chemical exchange processes can lead to complex NMR spectra that are difficult to interpret. These dynamic processes can cause signal broadening, coalescence, or the appearance of multiple resonances for a single carbonyl group.
Sample preparation and experimental conditions also play crucial roles in NMR carbonyl analysis. Factors such as solvent choice, temperature, and pH can significantly affect the chemical shifts and coupling patterns of carbonyl groups. Optimizing these parameters for each specific sample can be time-consuming and may require extensive method development.
Lastly, the quantitative analysis of carbonyl groups using NMR remains challenging. Variations in relaxation times and nuclear Overhauser effects can lead to inaccuracies in signal integration, making it difficult to determine the relative abundance of different carbonyl species in a sample.
Existing NMR Methods for Carbonyl Structure Elucidation
01 Spectroscopic analysis of carbonyl structures
Various spectroscopic techniques are employed to analyze carbonyl structures, including infrared spectroscopy, Raman spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy. These methods provide information about the vibrational modes, molecular structure, and chemical environment of carbonyl groups, allowing for detailed characterization and identification of carbonyl-containing compounds.- Spectroscopic analysis of carbonyl structures: Various spectroscopic techniques are employed to analyze carbonyl structures, including infrared spectroscopy, Raman spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy. These methods provide valuable information about the presence, position, and environment of carbonyl groups in molecules.
- Computational methods for carbonyl structure analysis: Advanced computational techniques, such as density functional theory (DFT) calculations and molecular dynamics simulations, are used to predict and analyze carbonyl structures. These methods help in understanding the electronic properties, reactivity, and conformational preferences of carbonyl-containing compounds.
- Chromatographic separation and analysis of carbonyl compounds: Chromatographic techniques, including gas chromatography (GC) and high-performance liquid chromatography (HPLC), are utilized for the separation and analysis of complex mixtures containing carbonyl compounds. These methods often involve derivatization steps to enhance detection and quantification of carbonyl structures.
- Mass spectrometry for carbonyl structure elucidation: Mass spectrometry techniques, such as electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI), are employed to determine the molecular mass and fragmentation patterns of carbonyl-containing compounds. This information is crucial for structural elucidation and identification of unknown carbonyl species.
- Chemical derivatization for carbonyl analysis: Chemical derivatization methods are used to modify carbonyl groups, making them more amenable to analysis or enhancing their detectability. Common derivatization reagents include 2,4-dinitrophenylhydrazine (DNPH) and o-phenylenediamine (OPD), which form stable adducts with carbonyl compounds for subsequent analysis.
02 Computational methods for carbonyl structure analysis
Advanced computational techniques are used to analyze and predict carbonyl structures. These include molecular modeling, density functional theory (DFT) calculations, and machine learning algorithms. Such methods help in understanding the electronic structure, reactivity, and properties of carbonyl compounds, aiding in the design and optimization of new molecules and materials.Expand Specific Solutions03 Chromatographic separation and analysis of carbonyl compounds
Chromatographic techniques, such as gas chromatography (GC) and high-performance liquid chromatography (HPLC), are utilized for the separation and analysis of complex mixtures containing carbonyl compounds. These methods, often coupled with mass spectrometry, enable the identification and quantification of individual carbonyl species in various samples, including environmental and biological matrices.Expand Specific Solutions04 Derivatization methods for carbonyl analysis
Chemical derivatization techniques are employed to enhance the detection and analysis of carbonyl compounds. These methods involve the reaction of carbonyl groups with specific reagents to form stable derivatives, which can be more easily detected and quantified using various analytical techniques. This approach is particularly useful for trace analysis and improving the selectivity of carbonyl compound detection.Expand Specific Solutions05 In situ and real-time monitoring of carbonyl structures
Advanced analytical techniques have been developed for in situ and real-time monitoring of carbonyl structures in various processes and environments. These include the use of specialized sensors, spectroscopic probes, and online analytical systems that allow for continuous monitoring of carbonyl formation, degradation, and reactivity in industrial processes, atmospheric chemistry, and biological systems.Expand Specific Solutions
Key Players in NMR Spectroscopy Industry
The analysis of carbonyl structures using NMR is a mature technology in the analytical chemistry field, with a competitive landscape dominated by established players. The market is in a growth phase, driven by increasing demand for structural analysis in pharmaceutical, chemical, and materials research. Key players include JEOL Ltd., Bruker BioSpin MRI GmbH, and Agilent Technologies, Inc., who offer advanced NMR spectrometers and solutions. These companies compete on instrument sensitivity, resolution, and software capabilities. Academic institutions like Xiamen University and Peking University contribute to research and method development. The market size is expanding due to growing applications in drug discovery, metabolomics, and materials science, with continuous technological advancements enhancing the capabilities of NMR analysis for carbonyl structures.
Schlumberger Technologies, Inc.
Technical Solution: Schlumberger Technologies, Inc. has adapted NMR technology for carbonyl structure analysis in the oil and gas industry. Their NMR logging tools, such as the CMR-Plus, can detect and characterize carbonyl groups in reservoir fluids and rock samples[15]. Schlumberger's proprietary interpretation software incorporates advanced algorithms for distinguishing carbonyl signals from complex hydrocarbon mixtures[16]. The company has also developed mobile NMR units for on-site analysis of carbonyl compounds in drilling fluids and production streams, enabling real-time decision-making in oilfield operations[17]. Additionally, Schlumberger's research centers utilize high-field NMR spectrometers for detailed structural analysis of carbonyl-containing compounds found in petroleum products[18].
Strengths: Specialized tools for in-situ carbonyl analysis in oil and gas applications, robust interpretation software for complex mixtures, and mobile NMR solutions. Weaknesses: Limited applicability outside the oil and gas sector, may require industry-specific expertise for optimal use.
JEOL Ltd.
Technical Solution: JEOL Ltd. has developed advanced NMR spectrometers specifically designed for carbonyl structure analysis. Their JNM-ECZ series incorporates high-field magnets (up to 1 GHz) and cryogenic probe technology, enabling enhanced sensitivity for detecting carbonyl groups[1]. The company's Delta software suite offers specialized pulse sequences and data processing algorithms optimized for carbonyl peak identification and quantification[2]. JEOL's systems also feature automated sample handling and temperature control, allowing for precise and reproducible carbonyl structure determinations across various sample types[3].
Strengths: High sensitivity and resolution for carbonyl detection, specialized software for data analysis, and automated sample handling. Weaknesses: High cost of equipment and maintenance, requires significant expertise to operate effectively.
Innovative NMR Pulse Sequences for Carbonyls
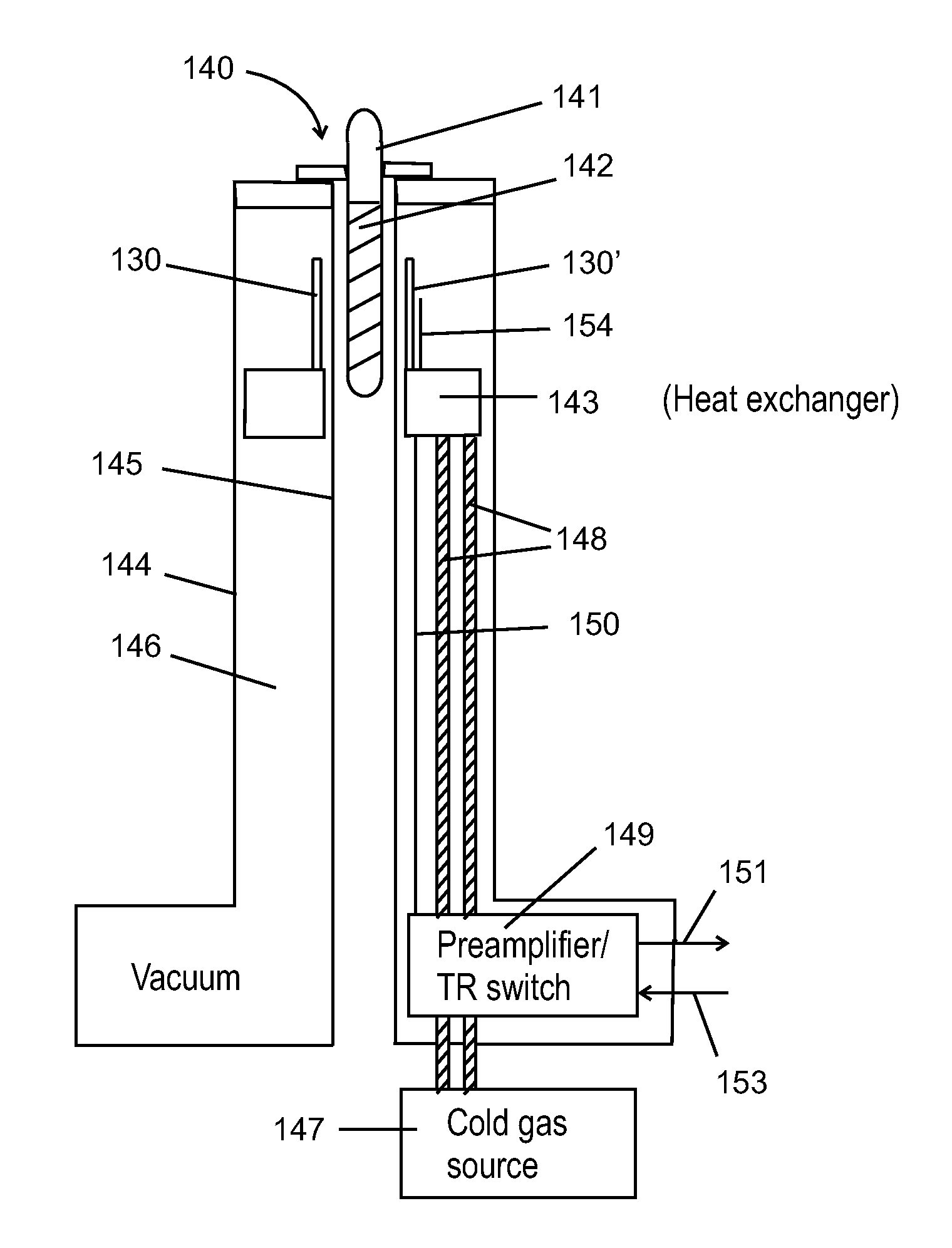
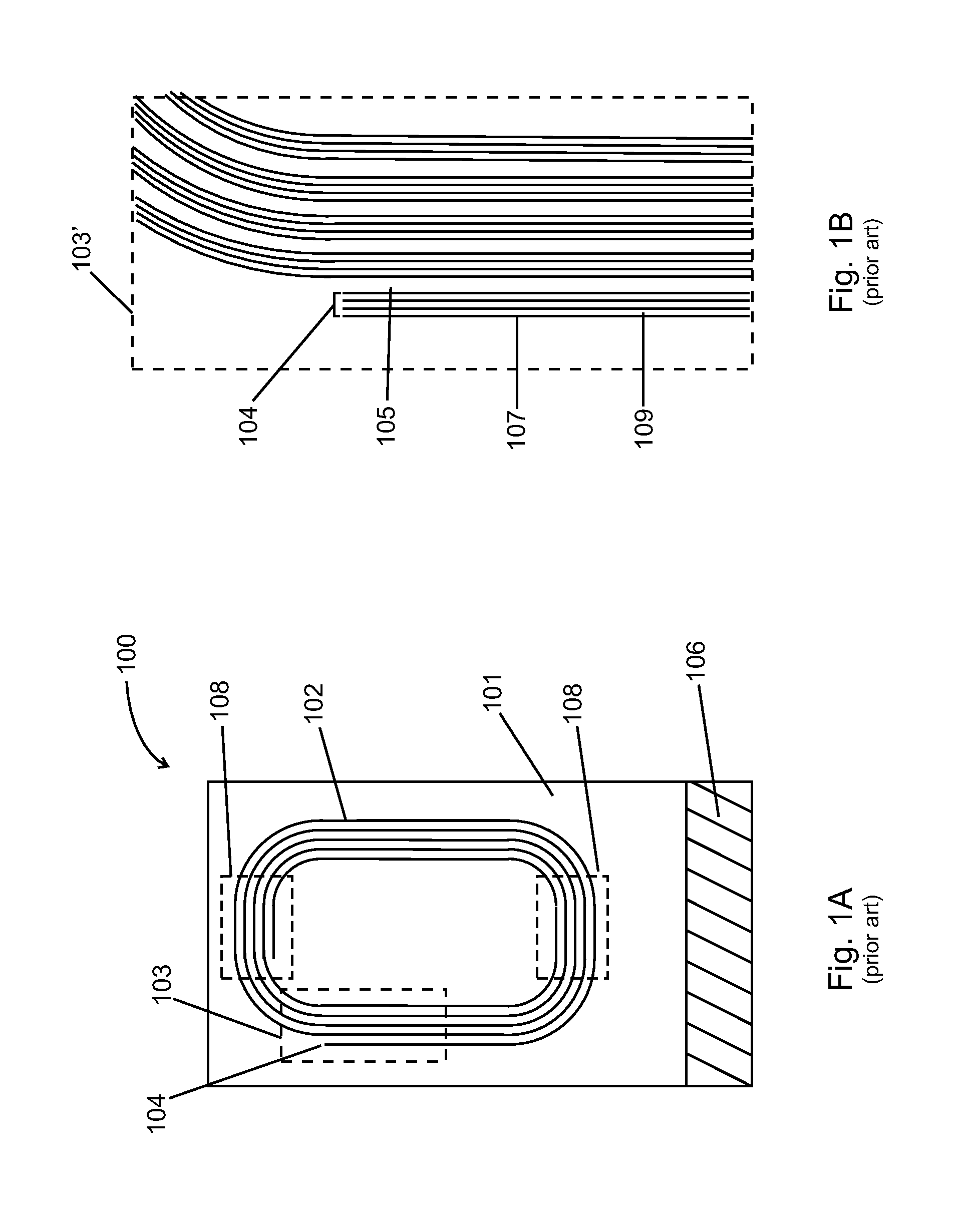
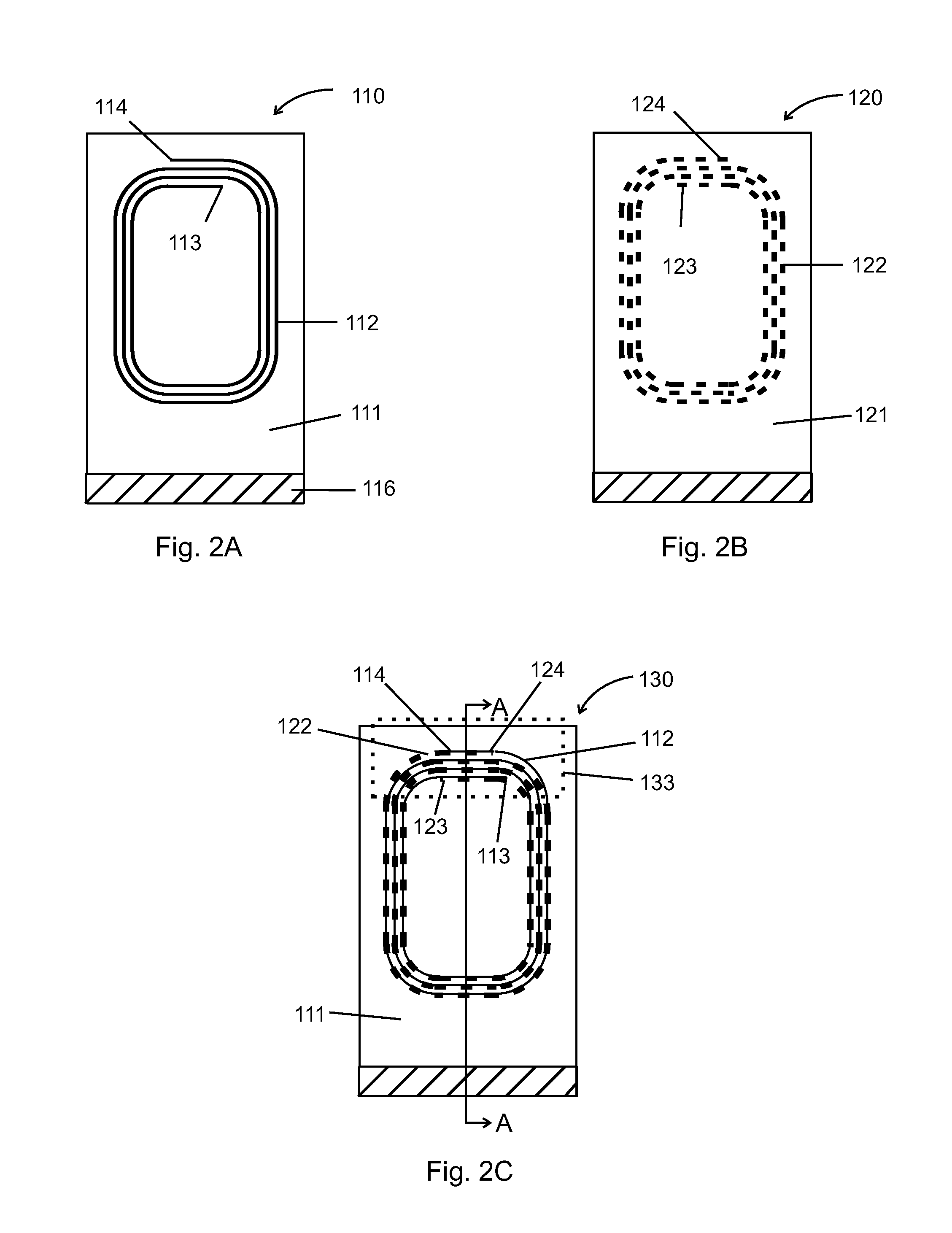
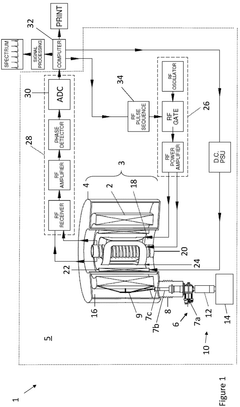
NMR spiral RF probe coil pair with low external electric field
PatentInactiveUS20100033184A1
Innovation
- The use of counter-wound spiral coil pairs, where each pair is wound on opposite sides of a dielectric layer with opposite directions, and adjusted to minimize electric field strength in the sample region, while maintaining a strong RF magnetic field, is implemented.
Nuclear magnetic resonance system
PatentActiveGB2597439A
Innovation
- A compact nuclear magnetic resonance system utilizing a superconducting magnetic coil cooled by a cryocooler, eliminating the need for liquid helium and achieving medium-to-high magnetic field strengths, allowing for a compact design suitable for laboratory benches.
Sample Preparation Techniques for NMR Carbonyl Analysis
Sample preparation is a critical step in the analysis of carbonyl structures using Nuclear Magnetic Resonance (NMR) spectroscopy. The quality and accuracy of the results heavily depend on proper sample handling and preparation techniques. One of the primary considerations is the choice of solvent. Deuterated solvents are typically used to avoid interference with the sample's NMR signals. For carbonyl compounds, common choices include deuterated chloroform (CDCl3), dimethyl sulfoxide (DMSO-d6), or acetone-d6, depending on the solubility and stability of the sample.
The concentration of the sample is another crucial factor. For most carbonyl compounds, a concentration range of 10-50 mM is suitable for 13C NMR analysis. However, this may vary depending on the specific carbonyl structure and the sensitivity of the NMR instrument. It's essential to ensure complete dissolution of the sample to obtain clear and well-resolved spectra.
Sample purity is paramount in NMR analysis. Any impurities can lead to additional peaks or distortions in the spectrum, complicating interpretation. Purification techniques such as recrystallization, column chromatography, or distillation may be necessary before NMR analysis. Additionally, filtration of the sample solution can help remove any undissolved particles that might affect the magnetic field homogeneity.
Temperature control during sample preparation and analysis is vital, especially for carbonyl compounds that may be sensitive to heat. Some carbonyl structures can undergo tautomerization or other structural changes at elevated temperatures, affecting the NMR results. Therefore, samples should be prepared at room temperature or under controlled cooling conditions when necessary.
For quantitative NMR analysis of carbonyl structures, the addition of an internal standard is often required. Tetramethylsilane (TMS) is a common choice, serving as a reference for chemical shift measurements. The internal standard should be chemically inert and not interfere with the sample's signals.
In cases where the carbonyl compound is air or moisture-sensitive, special handling techniques are required. Sample preparation may need to be carried out in an inert atmosphere, such as under nitrogen or argon gas. Sealed NMR tubes or J. Young NMR tubes can be used to maintain the sample's integrity during analysis.
For complex carbonyl structures or mixtures, two-dimensional NMR techniques may be employed. In such cases, sample preparation might involve isotopic labeling or the use of specific pulse sequences to enhance the detection of carbonyl groups. These advanced techniques often require more concentrated samples and longer acquisition times.
The concentration of the sample is another crucial factor. For most carbonyl compounds, a concentration range of 10-50 mM is suitable for 13C NMR analysis. However, this may vary depending on the specific carbonyl structure and the sensitivity of the NMR instrument. It's essential to ensure complete dissolution of the sample to obtain clear and well-resolved spectra.
Sample purity is paramount in NMR analysis. Any impurities can lead to additional peaks or distortions in the spectrum, complicating interpretation. Purification techniques such as recrystallization, column chromatography, or distillation may be necessary before NMR analysis. Additionally, filtration of the sample solution can help remove any undissolved particles that might affect the magnetic field homogeneity.
Temperature control during sample preparation and analysis is vital, especially for carbonyl compounds that may be sensitive to heat. Some carbonyl structures can undergo tautomerization or other structural changes at elevated temperatures, affecting the NMR results. Therefore, samples should be prepared at room temperature or under controlled cooling conditions when necessary.
For quantitative NMR analysis of carbonyl structures, the addition of an internal standard is often required. Tetramethylsilane (TMS) is a common choice, serving as a reference for chemical shift measurements. The internal standard should be chemically inert and not interfere with the sample's signals.
In cases where the carbonyl compound is air or moisture-sensitive, special handling techniques are required. Sample preparation may need to be carried out in an inert atmosphere, such as under nitrogen or argon gas. Sealed NMR tubes or J. Young NMR tubes can be used to maintain the sample's integrity during analysis.
For complex carbonyl structures or mixtures, two-dimensional NMR techniques may be employed. In such cases, sample preparation might involve isotopic labeling or the use of specific pulse sequences to enhance the detection of carbonyl groups. These advanced techniques often require more concentrated samples and longer acquisition times.
Data Processing and Interpretation Tools for NMR Spectra
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique for elucidating the structure of carbonyl compounds. To effectively analyze NMR spectra and extract valuable information about carbonyl structures, researchers rely on a variety of data processing and interpretation tools. These tools have evolved significantly over the years, enhancing the accuracy and efficiency of NMR analysis.
One of the primary data processing tools for NMR spectra is Fourier transformation. This mathematical technique converts the time-domain signal obtained from the NMR spectrometer into a frequency-domain spectrum. Modern NMR software packages automate this process, allowing for rapid conversion of raw data into interpretable spectra. Advanced algorithms have been developed to improve the quality of the Fourier transformation, reducing artifacts and enhancing spectral resolution.
Baseline correction is another crucial step in NMR data processing. Automated baseline correction algorithms help eliminate distortions and inconsistencies in the spectral baseline, ensuring accurate peak integration and quantification. These tools are particularly important for analyzing carbonyl compounds, as their signals can be affected by baseline distortions.
Peak picking and integration tools are essential for identifying and quantifying individual signals in NMR spectra. Advanced peak picking algorithms can automatically detect and label peaks, even in complex spectra with overlapping signals. Integration tools allow for accurate measurement of peak areas, which is crucial for determining the relative abundance of different carbonyl species in a sample.
Multiplet analysis tools assist in interpreting complex splitting patterns often observed in NMR spectra of carbonyl compounds. These tools can deconvolute overlapping multiplets, providing detailed information about coupling constants and helping to elucidate the molecular structure.
Two-dimensional NMR data processing tools have become increasingly important for analyzing complex carbonyl structures. These tools enable the visualization and interpretation of 2D NMR experiments such as COSY, HSQC, and HMBC, which provide valuable information about connectivity and spatial relationships between atoms in carbonyl compounds.
Spectral prediction software has emerged as a powerful aid in NMR interpretation. These tools use computational methods to predict NMR spectra based on proposed molecular structures, allowing researchers to compare experimental data with theoretical predictions and refine their structural hypotheses.
Database searching and spectral matching tools have revolutionized the process of structure elucidation. By comparing experimental NMR data with extensive libraries of known compounds, these tools can rapidly identify or suggest possible structures for unknown carbonyl compounds.
As the field of NMR spectroscopy continues to advance, machine learning and artificial intelligence approaches are being integrated into data processing and interpretation tools. These cutting-edge techniques promise to further enhance the speed and accuracy of carbonyl structure analysis using NMR.
One of the primary data processing tools for NMR spectra is Fourier transformation. This mathematical technique converts the time-domain signal obtained from the NMR spectrometer into a frequency-domain spectrum. Modern NMR software packages automate this process, allowing for rapid conversion of raw data into interpretable spectra. Advanced algorithms have been developed to improve the quality of the Fourier transformation, reducing artifacts and enhancing spectral resolution.
Baseline correction is another crucial step in NMR data processing. Automated baseline correction algorithms help eliminate distortions and inconsistencies in the spectral baseline, ensuring accurate peak integration and quantification. These tools are particularly important for analyzing carbonyl compounds, as their signals can be affected by baseline distortions.
Peak picking and integration tools are essential for identifying and quantifying individual signals in NMR spectra. Advanced peak picking algorithms can automatically detect and label peaks, even in complex spectra with overlapping signals. Integration tools allow for accurate measurement of peak areas, which is crucial for determining the relative abundance of different carbonyl species in a sample.
Multiplet analysis tools assist in interpreting complex splitting patterns often observed in NMR spectra of carbonyl compounds. These tools can deconvolute overlapping multiplets, providing detailed information about coupling constants and helping to elucidate the molecular structure.
Two-dimensional NMR data processing tools have become increasingly important for analyzing complex carbonyl structures. These tools enable the visualization and interpretation of 2D NMR experiments such as COSY, HSQC, and HMBC, which provide valuable information about connectivity and spatial relationships between atoms in carbonyl compounds.
Spectral prediction software has emerged as a powerful aid in NMR interpretation. These tools use computational methods to predict NMR spectra based on proposed molecular structures, allowing researchers to compare experimental data with theoretical predictions and refine their structural hypotheses.
Database searching and spectral matching tools have revolutionized the process of structure elucidation. By comparing experimental NMR data with extensive libraries of known compounds, these tools can rapidly identify or suggest possible structures for unknown carbonyl compounds.
As the field of NMR spectroscopy continues to advance, machine learning and artificial intelligence approaches are being integrated into data processing and interpretation tools. These cutting-edge techniques promise to further enhance the speed and accuracy of carbonyl structure analysis using NMR.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!