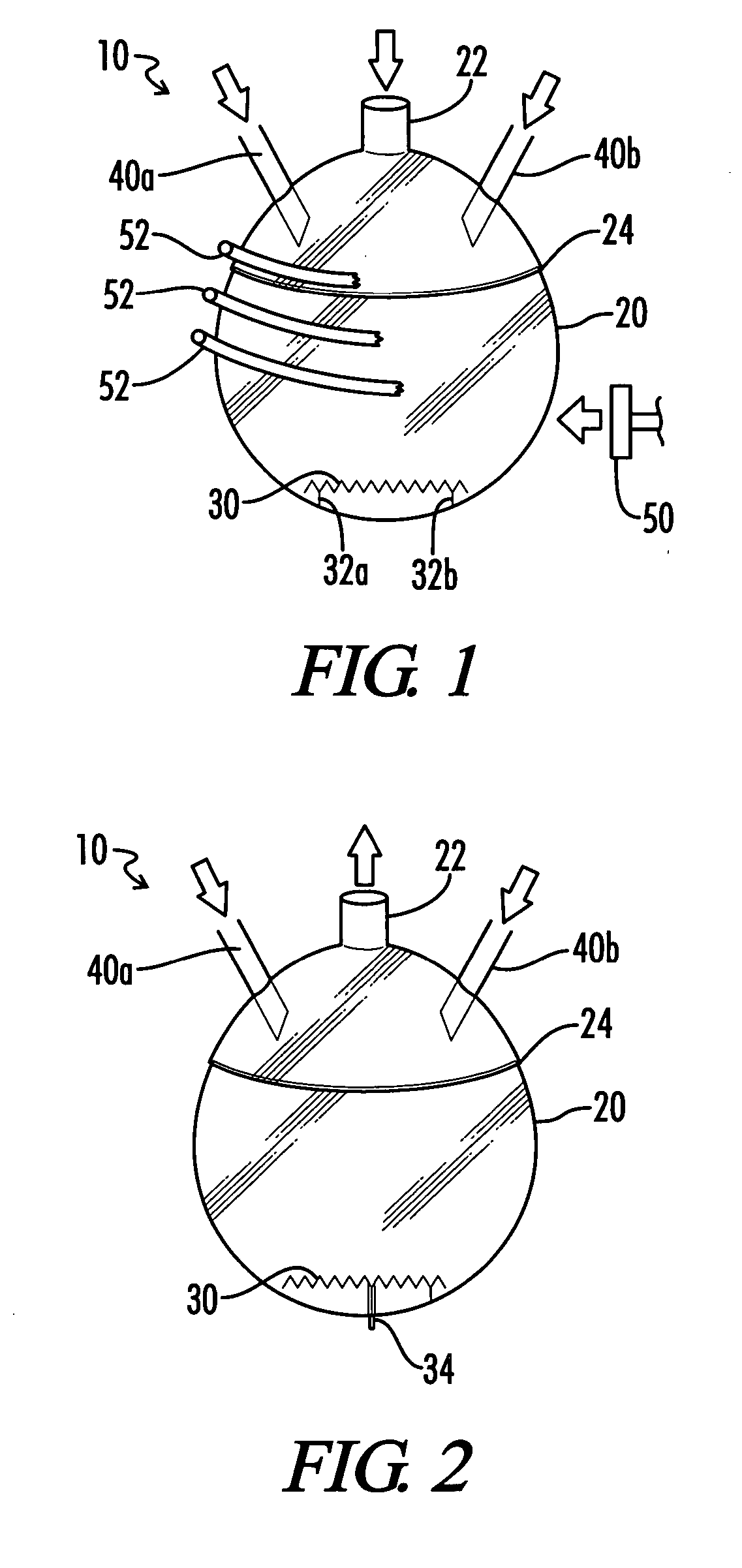
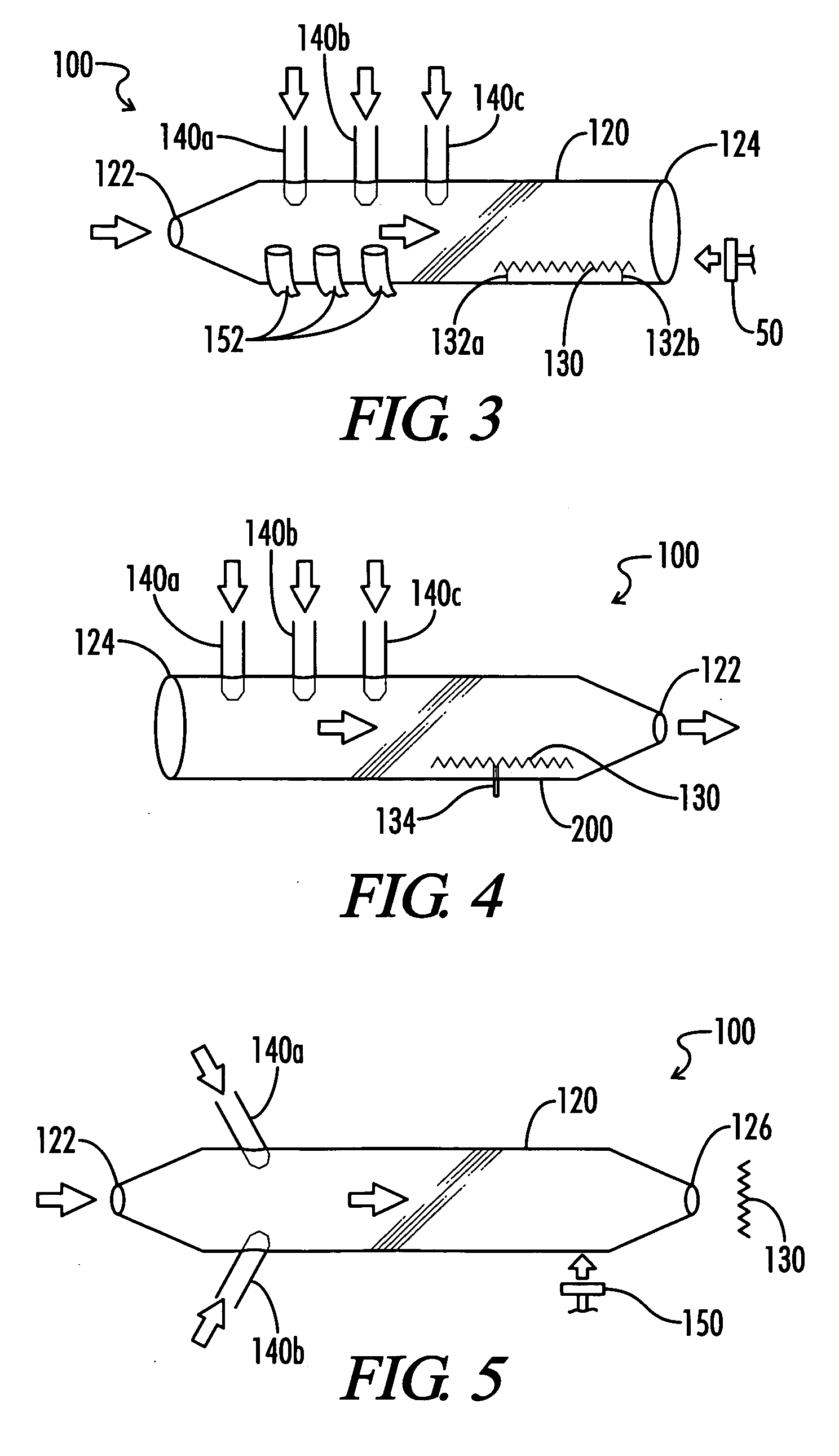
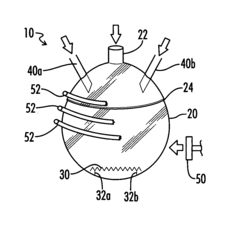
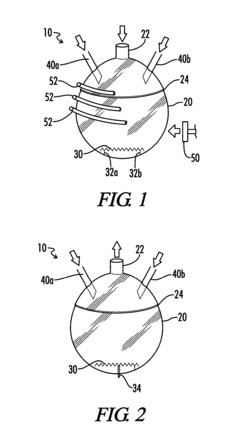
How to Utilize Carbonyl Chemistry for Greater Environmental Impact?
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Chemistry Background and Objectives
Carbonyl chemistry has been a cornerstone of organic synthesis for over a century, playing a crucial role in the development of pharmaceuticals, materials, and industrial processes. This branch of chemistry focuses on compounds containing the carbonyl group (C=O), which is characterized by its unique reactivity and versatility. The evolution of carbonyl chemistry has been marked by significant breakthroughs in reaction mechanisms, synthetic methodologies, and applications across various industries.
In recent years, there has been a growing emphasis on leveraging carbonyl chemistry to address environmental challenges. This shift is driven by the increasing global awareness of sustainability issues and the urgent need for greener chemical processes. The objective of this technical research is to explore innovative ways to utilize carbonyl chemistry for greater environmental impact, aligning with the principles of green chemistry and sustainable development.
One of the primary goals is to develop more efficient and environmentally friendly synthetic routes that exploit the reactivity of carbonyl compounds. This includes the design of atom-economical reactions, the use of renewable feedstocks, and the minimization of waste products. Additionally, there is a focus on harnessing carbonyl chemistry for the development of biodegradable materials, which could potentially replace persistent plastics and reduce environmental pollution.
Another key objective is to investigate the role of carbonyl chemistry in carbon capture and utilization technologies. The unique properties of carbonyl compounds make them promising candidates for CO2 fixation and conversion into value-added products. This research direction aligns with global efforts to mitigate climate change and reduce greenhouse gas emissions.
Furthermore, the application of carbonyl chemistry in the field of environmental remediation is being explored. This includes the development of novel adsorbents and catalysts for the removal of pollutants from water and air, as well as the design of sensors for environmental monitoring based on carbonyl-containing molecules.
The technical landscape of carbonyl chemistry is rapidly evolving, with emerging trends such as photocatalysis, electrochemistry, and biocatalysis offering new avenues for environmentally benign transformations. These approaches aim to reduce energy consumption, eliminate the need for toxic reagents, and enable reactions under milder conditions.
As we delve deeper into the potential of carbonyl chemistry for environmental applications, it is crucial to consider the entire lifecycle of products and processes. This holistic approach encompasses not only the immediate reactions and their outcomes but also the long-term environmental impact of the materials and technologies developed through carbonyl chemistry.
In recent years, there has been a growing emphasis on leveraging carbonyl chemistry to address environmental challenges. This shift is driven by the increasing global awareness of sustainability issues and the urgent need for greener chemical processes. The objective of this technical research is to explore innovative ways to utilize carbonyl chemistry for greater environmental impact, aligning with the principles of green chemistry and sustainable development.
One of the primary goals is to develop more efficient and environmentally friendly synthetic routes that exploit the reactivity of carbonyl compounds. This includes the design of atom-economical reactions, the use of renewable feedstocks, and the minimization of waste products. Additionally, there is a focus on harnessing carbonyl chemistry for the development of biodegradable materials, which could potentially replace persistent plastics and reduce environmental pollution.
Another key objective is to investigate the role of carbonyl chemistry in carbon capture and utilization technologies. The unique properties of carbonyl compounds make them promising candidates for CO2 fixation and conversion into value-added products. This research direction aligns with global efforts to mitigate climate change and reduce greenhouse gas emissions.
Furthermore, the application of carbonyl chemistry in the field of environmental remediation is being explored. This includes the development of novel adsorbents and catalysts for the removal of pollutants from water and air, as well as the design of sensors for environmental monitoring based on carbonyl-containing molecules.
The technical landscape of carbonyl chemistry is rapidly evolving, with emerging trends such as photocatalysis, electrochemistry, and biocatalysis offering new avenues for environmentally benign transformations. These approaches aim to reduce energy consumption, eliminate the need for toxic reagents, and enable reactions under milder conditions.
As we delve deeper into the potential of carbonyl chemistry for environmental applications, it is crucial to consider the entire lifecycle of products and processes. This holistic approach encompasses not only the immediate reactions and their outcomes but also the long-term environmental impact of the materials and technologies developed through carbonyl chemistry.
Market Demand for Green Carbonyl Reactions
The market demand for green carbonyl reactions has been steadily increasing in recent years, driven by growing environmental concerns and stricter regulations on chemical processes. Industries across various sectors, including pharmaceuticals, agrochemicals, and materials science, are actively seeking more sustainable alternatives to traditional carbonyl chemistry methods.
In the pharmaceutical industry, there is a significant push towards greener synthesis routes for active pharmaceutical ingredients (APIs). Many APIs contain carbonyl groups, and their production often involves carbonyl chemistry. The demand for environmentally friendly carbonyl reactions in this sector is particularly high, as pharmaceutical companies aim to reduce their carbon footprint and meet sustainability goals.
The agrochemical industry is another major driver of demand for green carbonyl reactions. Pesticides and herbicides frequently incorporate carbonyl functionalities, and there is increasing pressure to develop more eco-friendly production methods. Consumers and regulatory bodies are demanding agricultural products with lower environmental impact, pushing manufacturers to adopt greener synthesis techniques.
In the materials science sector, the development of biodegradable polymers and sustainable packaging materials has created a new market for green carbonyl chemistry. Many of these materials rely on carbonyl-containing monomers, and there is a growing need for environmentally benign methods to produce and modify these compounds.
The fine chemicals industry is also experiencing a shift towards greener processes, with carbonyl chemistry playing a crucial role in the synthesis of various specialty chemicals. Companies in this sector are investing in research and development of sustainable carbonyl reactions to meet the increasing demand for eco-friendly products.
The global market for green chemistry, which encompasses environmentally friendly carbonyl reactions, is projected to grow significantly in the coming years. This growth is fueled by both consumer preferences for sustainable products and government regulations promoting cleaner industrial processes.
Academic research in green carbonyl chemistry has seen a surge in recent years, indicating a strong potential for innovation and new market opportunities. Universities and research institutions are collaborating with industry partners to develop novel catalysts and reaction conditions that enable more efficient and environmentally friendly carbonyl transformations.
As the world moves towards a circular economy model, there is an increasing demand for carbonyl reactions that can be used in recycling and upcycling processes. This includes the development of methods to break down carbonyl-containing polymers and convert waste materials into valuable chemical feedstocks.
The market for green carbonyl reactions is not limited to developed economies. Emerging markets, particularly in Asia and South America, are showing a growing interest in sustainable chemical processes as they balance industrial growth with environmental protection. This global demand is creating new opportunities for companies specializing in green chemistry technologies.
In the pharmaceutical industry, there is a significant push towards greener synthesis routes for active pharmaceutical ingredients (APIs). Many APIs contain carbonyl groups, and their production often involves carbonyl chemistry. The demand for environmentally friendly carbonyl reactions in this sector is particularly high, as pharmaceutical companies aim to reduce their carbon footprint and meet sustainability goals.
The agrochemical industry is another major driver of demand for green carbonyl reactions. Pesticides and herbicides frequently incorporate carbonyl functionalities, and there is increasing pressure to develop more eco-friendly production methods. Consumers and regulatory bodies are demanding agricultural products with lower environmental impact, pushing manufacturers to adopt greener synthesis techniques.
In the materials science sector, the development of biodegradable polymers and sustainable packaging materials has created a new market for green carbonyl chemistry. Many of these materials rely on carbonyl-containing monomers, and there is a growing need for environmentally benign methods to produce and modify these compounds.
The fine chemicals industry is also experiencing a shift towards greener processes, with carbonyl chemistry playing a crucial role in the synthesis of various specialty chemicals. Companies in this sector are investing in research and development of sustainable carbonyl reactions to meet the increasing demand for eco-friendly products.
The global market for green chemistry, which encompasses environmentally friendly carbonyl reactions, is projected to grow significantly in the coming years. This growth is fueled by both consumer preferences for sustainable products and government regulations promoting cleaner industrial processes.
Academic research in green carbonyl chemistry has seen a surge in recent years, indicating a strong potential for innovation and new market opportunities. Universities and research institutions are collaborating with industry partners to develop novel catalysts and reaction conditions that enable more efficient and environmentally friendly carbonyl transformations.
As the world moves towards a circular economy model, there is an increasing demand for carbonyl reactions that can be used in recycling and upcycling processes. This includes the development of methods to break down carbonyl-containing polymers and convert waste materials into valuable chemical feedstocks.
The market for green carbonyl reactions is not limited to developed economies. Emerging markets, particularly in Asia and South America, are showing a growing interest in sustainable chemical processes as they balance industrial growth with environmental protection. This global demand is creating new opportunities for companies specializing in green chemistry technologies.
Current Challenges in Sustainable Carbonyl Chemistry
Sustainable carbonyl chemistry faces several significant challenges in its pursuit of greater environmental impact. One of the primary obstacles is the reliance on fossil fuel-derived feedstocks for many carbonyl compounds. This dependence not only contributes to carbon emissions but also raises concerns about long-term sustainability and resource depletion. The transition to renewable feedstocks, while promising, presents its own set of technical and economic hurdles.
Another major challenge lies in the energy-intensive nature of many carbonyl reactions. Traditional synthesis methods often require high temperatures and pressures, leading to substantial energy consumption and associated environmental impacts. Developing energy-efficient processes that can operate under milder conditions remains a key focus for researchers in the field.
The use of toxic or hazardous reagents and solvents in carbonyl chemistry also poses significant environmental and safety concerns. Many conventional reactions rely on harmful substances that can have detrimental effects on ecosystems and human health. Finding greener alternatives that maintain or improve reaction efficiency is crucial for advancing sustainable practices in this area.
Waste generation and management represent another critical challenge. Carbonyl reactions frequently produce byproducts and require extensive purification steps, resulting in substantial waste streams. Improving atom economy, developing more selective reactions, and implementing effective recycling strategies are essential for minimizing environmental impact.
The scalability of sustainable carbonyl processes presents a significant hurdle in industrial applications. Many environmentally friendly methods developed in laboratory settings face difficulties when scaled up to commercial production levels. Overcoming these challenges requires innovative engineering solutions and process optimizations to maintain efficiency and sustainability at larger scales.
Additionally, the economic viability of sustainable carbonyl chemistry remains a concern. Green alternatives often come with higher costs, making them less competitive in the market. Balancing environmental benefits with economic feasibility is crucial for widespread adoption of sustainable practices in the industry.
Lastly, the complexity of life cycle assessments for carbonyl compounds and their derivatives poses a challenge in accurately quantifying environmental impacts. Developing comprehensive and standardized methods for evaluating the sustainability of different processes and products is essential for informed decision-making and continuous improvement in the field.
Another major challenge lies in the energy-intensive nature of many carbonyl reactions. Traditional synthesis methods often require high temperatures and pressures, leading to substantial energy consumption and associated environmental impacts. Developing energy-efficient processes that can operate under milder conditions remains a key focus for researchers in the field.
The use of toxic or hazardous reagents and solvents in carbonyl chemistry also poses significant environmental and safety concerns. Many conventional reactions rely on harmful substances that can have detrimental effects on ecosystems and human health. Finding greener alternatives that maintain or improve reaction efficiency is crucial for advancing sustainable practices in this area.
Waste generation and management represent another critical challenge. Carbonyl reactions frequently produce byproducts and require extensive purification steps, resulting in substantial waste streams. Improving atom economy, developing more selective reactions, and implementing effective recycling strategies are essential for minimizing environmental impact.
The scalability of sustainable carbonyl processes presents a significant hurdle in industrial applications. Many environmentally friendly methods developed in laboratory settings face difficulties when scaled up to commercial production levels. Overcoming these challenges requires innovative engineering solutions and process optimizations to maintain efficiency and sustainability at larger scales.
Additionally, the economic viability of sustainable carbonyl chemistry remains a concern. Green alternatives often come with higher costs, making them less competitive in the market. Balancing environmental benefits with economic feasibility is crucial for widespread adoption of sustainable practices in the industry.
Lastly, the complexity of life cycle assessments for carbonyl compounds and their derivatives poses a challenge in accurately quantifying environmental impacts. Developing comprehensive and standardized methods for evaluating the sustainability of different processes and products is essential for informed decision-making and continuous improvement in the field.
Existing Green Carbonyl Reaction Methodologies
01 Environmental impact assessment of carbonyl compounds
Carbonyl compounds, including aldehydes and ketones, can have significant environmental impacts. This point focuses on methods and systems for assessing the environmental effects of these compounds, including their persistence, bioaccumulation, and toxicity in various ecosystems. It also covers strategies for monitoring and mitigating the release of carbonyl compounds into the environment.- Environmental impact assessment of carbonyl compounds: Carbonyl compounds, including aldehydes and ketones, can have significant environmental impacts. This point focuses on methods and systems for assessing the environmental effects of these compounds, including their potential contributions to air and water pollution, as well as their role in atmospheric chemistry and climate change.
- Green chemistry approaches for carbonyl reactions: This category covers environmentally friendly approaches to carbonyl chemistry, including the development of sustainable catalysts, solvent-free reactions, and the use of renewable resources as starting materials. These methods aim to reduce the environmental footprint of carbonyl reactions in industrial and research settings.
- Carbonyl compound degradation and remediation: This point addresses techniques and technologies for the degradation and remediation of carbonyl compounds in the environment. It includes biological treatment methods, advanced oxidation processes, and other innovative approaches to mitigate the environmental impact of these chemicals in soil, water, and air.
- Monitoring and detection of carbonyl pollutants: This category focuses on methods and devices for monitoring and detecting carbonyl compounds in various environmental matrices. It includes the development of sensitive and selective sensors, real-time monitoring systems, and data analysis techniques to assess the presence and concentration of these pollutants in the environment.
- Life cycle assessment of carbonyl-based products: This point covers the life cycle assessment of products and processes involving carbonyl compounds. It includes methodologies for evaluating the environmental impact of these chemicals from production to disposal, considering factors such as energy consumption, resource depletion, and emissions throughout their lifecycle.
02 Green chemistry approaches for carbonyl reactions
This point addresses the development of environmentally friendly methods for carbonyl chemistry. It includes the use of renewable resources, catalysts, and solvents that reduce the environmental footprint of carbonyl reactions. The focus is on improving reaction efficiency, reducing waste, and minimizing the use of hazardous substances in carbonyl compound synthesis and transformations.Expand Specific Solutions03 Atmospheric chemistry of carbonyl compounds
Carbonyl compounds play a crucial role in atmospheric chemistry, affecting air quality and climate change. This point covers research on the formation, transformation, and degradation of carbonyl compounds in the atmosphere. It includes studies on their role in smog formation, ozone depletion, and the generation of secondary organic aerosols.Expand Specific Solutions04 Biodegradation and remediation of carbonyl pollutants
This point focuses on the natural breakdown of carbonyl compounds in the environment and the development of remediation technologies. It covers microbial degradation pathways, enzymatic processes, and engineered systems for the removal of carbonyl pollutants from soil, water, and air. The aim is to reduce the environmental persistence and toxicity of these compounds.Expand Specific Solutions05 Life cycle assessment of carbonyl-based products
This point addresses the comprehensive environmental impact analysis of products and processes involving carbonyl chemistry. It includes the evaluation of raw material extraction, manufacturing processes, use phase, and end-of-life disposal or recycling. The goal is to identify hotspots in the life cycle and propose improvements to reduce the overall environmental footprint of carbonyl-based products.Expand Specific Solutions
Key Players in Green Carbonyl Chemistry
The carbonyl chemistry market is in a growth phase, driven by increasing environmental concerns and sustainable chemistry initiatives. The market size is expanding, with applications spanning various industries. Technological maturity varies across different carbonyl processes, with some established methods and emerging eco-friendly approaches. Key players like BASF Corp., Sumitomo Chemical Co., Ltd., and Mitsubishi Gas Chemical Co., Inc. are investing in R&D to develop greener carbonyl chemistry solutions. Academic institutions such as Kobe University and East China Normal University are contributing to fundamental research, while companies like Directa Plus SpA and Ingelia SL are exploring innovative applications in materials science and renewable energy, respectively.
Celanese International Corp.
Technical Solution: Celanese has made significant strides in utilizing carbonyl chemistry for environmental benefits. They've developed a proprietary technology called TCX® for producing ethanol from syngas, which can be derived from various feedstocks including biomass and municipal solid waste[5]. This process has a lower carbon footprint compared to traditional ethanol production methods. Celanese has also focused on developing bio-based acetyl intermediates, which are key building blocks in many industrial processes. Their GreenAcetyl™ technology allows for the production of acetic acid and vinyl acetate monomer using renewable feedstocks, potentially reducing greenhouse gas emissions by up to 50%[6].
Strengths: Strong expertise in acetyl chemistry, innovative process technologies. Weaknesses: Dependence on volatile raw material prices, potential competition from bio-based alternatives.
BASF Corp.
Technical Solution: BASF has developed innovative carbonyl chemistry processes for greater environmental impact. Their eCO2 technology uses carbon dioxide as a raw material for polyurethane production, reducing reliance on fossil fuels[1]. They've also pioneered a novel process for producing acrylic acid from ethylene and CO2, potentially cutting greenhouse gas emissions by up to 30%[2]. Additionally, BASF's biomass balance approach incorporates renewable raw materials into existing production processes, further reducing carbon footprint[3]. Their commitment to green chemistry is evident in their development of water-based polyurethane dispersions and bio-based succinic acid production[4].
Strengths: Global leader in chemical innovation, extensive R&D capabilities, strong focus on sustainability. Weaknesses: High investment costs for new technologies, potential regulatory challenges in different markets.
Innovative Catalysts for Sustainable Carbonyl Transformations
Continuous process and apparatus for the production of catalyst-coated support materials
PatentInactiveUS20070037700A1
Innovation
- A continuous process using a decomposable metal-containing moiety, such as metal carbonyls, is fed into a reactor vessel where it decomposes to produce nano-scale catalyst particles that can be directly deposited on a support at moderate temperatures and pressures, eliminating the need for carrier particles and extreme conditions.
Continuous process and apparatus for the production of engineered catalyst materials
PatentInactiveUS20070036912A1
Innovation
- A continuous process and apparatus that decompose metal-containing moieties, such as metal carbonyls, in a reactor vessel to produce nano-scale catalyst particles with controlled size and composition, allowing for precise deposition on supports without extreme conditions, using energy sources like heat, microwave, or ultraviolet light.
Life Cycle Assessment of Carbonyl Reactions
Life Cycle Assessment (LCA) of carbonyl reactions is a crucial tool for evaluating the environmental impact of these chemical processes. This comprehensive analysis encompasses the entire lifecycle of carbonyl compounds, from raw material extraction to final disposal, providing valuable insights into their ecological footprint.
The assessment begins with the sourcing of raw materials, typically involving the extraction of fossil fuels or biomass. This stage accounts for energy consumption, resource depletion, and emissions associated with mining or harvesting activities. The transportation of these materials to processing facilities is also factored into the analysis, considering fuel consumption and related emissions.
The production phase of carbonyl compounds is a key focus of the LCA. This stage involves various chemical reactions, often requiring significant energy inputs and potentially generating harmful byproducts. The assessment quantifies energy usage, water consumption, and emissions of greenhouse gases and other pollutants. It also evaluates the efficiency of catalysts and solvents used in these reactions, as well as the potential for recycling or reusing these materials.
The use phase of carbonyl compounds varies widely depending on their application. In some cases, such as in the production of pharmaceuticals or polymers, the compounds are incorporated into final products. In others, they may be used as intermediates in further chemical processes. The LCA considers the environmental impact of these applications, including any emissions or waste generated during use.
End-of-life considerations form a critical component of the assessment. This includes the disposal or recycling of products containing carbonyl compounds, as well as the management of waste generated during production processes. The potential for biodegradation, incineration, or landfilling is evaluated, along with associated environmental impacts such as soil and water contamination or greenhouse gas emissions.
Throughout the LCA, various environmental impact categories are assessed, including global warming potential, ozone depletion, acidification, eutrophication, and human toxicity. These metrics provide a comprehensive view of the ecological consequences of carbonyl chemistry across different environmental domains.
The results of the LCA can be used to identify hotspots in the lifecycle where environmental impacts are most significant. This information is invaluable for researchers and industry professionals seeking to optimize carbonyl reactions for greater sustainability. It can guide efforts to develop greener synthesis routes, improve energy efficiency, reduce waste generation, and explore alternative raw materials or catalysts.
Moreover, the LCA findings can inform policy decisions and regulatory frameworks aimed at mitigating the environmental impact of chemical processes. By providing a quantitative basis for comparing different production methods and applications of carbonyl compounds, LCA supports evidence-based decision-making in both industry and governance.
The assessment begins with the sourcing of raw materials, typically involving the extraction of fossil fuels or biomass. This stage accounts for energy consumption, resource depletion, and emissions associated with mining or harvesting activities. The transportation of these materials to processing facilities is also factored into the analysis, considering fuel consumption and related emissions.
The production phase of carbonyl compounds is a key focus of the LCA. This stage involves various chemical reactions, often requiring significant energy inputs and potentially generating harmful byproducts. The assessment quantifies energy usage, water consumption, and emissions of greenhouse gases and other pollutants. It also evaluates the efficiency of catalysts and solvents used in these reactions, as well as the potential for recycling or reusing these materials.
The use phase of carbonyl compounds varies widely depending on their application. In some cases, such as in the production of pharmaceuticals or polymers, the compounds are incorporated into final products. In others, they may be used as intermediates in further chemical processes. The LCA considers the environmental impact of these applications, including any emissions or waste generated during use.
End-of-life considerations form a critical component of the assessment. This includes the disposal or recycling of products containing carbonyl compounds, as well as the management of waste generated during production processes. The potential for biodegradation, incineration, or landfilling is evaluated, along with associated environmental impacts such as soil and water contamination or greenhouse gas emissions.
Throughout the LCA, various environmental impact categories are assessed, including global warming potential, ozone depletion, acidification, eutrophication, and human toxicity. These metrics provide a comprehensive view of the ecological consequences of carbonyl chemistry across different environmental domains.
The results of the LCA can be used to identify hotspots in the lifecycle where environmental impacts are most significant. This information is invaluable for researchers and industry professionals seeking to optimize carbonyl reactions for greater sustainability. It can guide efforts to develop greener synthesis routes, improve energy efficiency, reduce waste generation, and explore alternative raw materials or catalysts.
Moreover, the LCA findings can inform policy decisions and regulatory frameworks aimed at mitigating the environmental impact of chemical processes. By providing a quantitative basis for comparing different production methods and applications of carbonyl compounds, LCA supports evidence-based decision-making in both industry and governance.
Policy Implications for Green Chemistry Adoption
The adoption of green chemistry principles, particularly in the realm of carbonyl chemistry, requires a comprehensive policy framework to drive widespread implementation and maximize environmental impact. Policymakers must consider a multi-faceted approach that addresses both incentives and regulations to encourage the transition towards more sustainable chemical processes.
One key policy implication is the need for increased funding and support for research and development in green carbonyl chemistry. Governments should allocate resources to academic institutions and private sector initiatives focused on developing innovative, environmentally friendly carbonyl reactions and processes. This investment can accelerate the discovery of novel catalysts, reaction pathways, and sustainable solvents that reduce waste and energy consumption.
Regulatory frameworks play a crucial role in promoting green chemistry adoption. Policymakers should consider implementing stricter environmental standards for chemical manufacturing, with a specific focus on carbonyl chemistry applications. These regulations could include limits on toxic byproduct generation, requirements for solvent recycling, and mandates for energy-efficient processes. Such measures would create a level playing field and incentivize companies to invest in greener technologies.
Tax incentives and subsidies can be powerful tools to encourage the adoption of green carbonyl chemistry practices. Governments could offer tax credits or grants to companies that implement sustainable carbonyl reactions or invest in eco-friendly equipment and infrastructure. These financial incentives can help offset the initial costs associated with transitioning to greener processes and make them more economically viable for businesses.
Education and training programs are essential for building a workforce capable of implementing green carbonyl chemistry principles. Policymakers should support the development of specialized curricula in universities and vocational institutions, focusing on sustainable chemical processes and green chemistry techniques. Additionally, continuing education programs for industry professionals can help disseminate knowledge and best practices throughout the chemical sector.
International cooperation and harmonization of green chemistry standards are crucial for achieving global environmental impact. Policymakers should work towards establishing common guidelines and metrics for assessing the sustainability of carbonyl chemistry processes across borders. This could involve collaborative research initiatives, knowledge-sharing platforms, and joint policy development among nations to address global environmental challenges collectively.
Lastly, policymakers must consider the broader implications of green carbonyl chemistry adoption on supply chains and industrial ecosystems. Policies should be designed to support the development of sustainable chemical feedstocks, promote circular economy principles in chemical manufacturing, and encourage the integration of green chemistry practices throughout the value chain. This holistic approach can lead to more significant environmental benefits and foster a more resilient and sustainable chemical industry.
One key policy implication is the need for increased funding and support for research and development in green carbonyl chemistry. Governments should allocate resources to academic institutions and private sector initiatives focused on developing innovative, environmentally friendly carbonyl reactions and processes. This investment can accelerate the discovery of novel catalysts, reaction pathways, and sustainable solvents that reduce waste and energy consumption.
Regulatory frameworks play a crucial role in promoting green chemistry adoption. Policymakers should consider implementing stricter environmental standards for chemical manufacturing, with a specific focus on carbonyl chemistry applications. These regulations could include limits on toxic byproduct generation, requirements for solvent recycling, and mandates for energy-efficient processes. Such measures would create a level playing field and incentivize companies to invest in greener technologies.
Tax incentives and subsidies can be powerful tools to encourage the adoption of green carbonyl chemistry practices. Governments could offer tax credits or grants to companies that implement sustainable carbonyl reactions or invest in eco-friendly equipment and infrastructure. These financial incentives can help offset the initial costs associated with transitioning to greener processes and make them more economically viable for businesses.
Education and training programs are essential for building a workforce capable of implementing green carbonyl chemistry principles. Policymakers should support the development of specialized curricula in universities and vocational institutions, focusing on sustainable chemical processes and green chemistry techniques. Additionally, continuing education programs for industry professionals can help disseminate knowledge and best practices throughout the chemical sector.
International cooperation and harmonization of green chemistry standards are crucial for achieving global environmental impact. Policymakers should work towards establishing common guidelines and metrics for assessing the sustainability of carbonyl chemistry processes across borders. This could involve collaborative research initiatives, knowledge-sharing platforms, and joint policy development among nations to address global environmental challenges collectively.
Lastly, policymakers must consider the broader implications of green carbonyl chemistry adoption on supply chains and industrial ecosystems. Policies should be designed to support the development of sustainable chemical feedstocks, promote circular economy principles in chemical manufacturing, and encourage the integration of green chemistry practices throughout the value chain. This holistic approach can lead to more significant environmental benefits and foster a more resilient and sustainable chemical industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!