Carbonyl-Based Processes Driving Sustainable Production
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Process Evolution
Carbonyl-based processes have undergone significant evolution since their inception in the early 20th century. The timeline of this evolution can be traced through several key stages, each marked by technological advancements and shifts in industrial priorities.
The foundation of carbonyl chemistry was laid in the 1920s with the discovery of the oxo synthesis by Otto Roelen. This breakthrough paved the way for the industrial production of aldehydes and alcohols from olefins and syngas. The 1930s and 1940s saw the development of the Reppe chemistry, which expanded the scope of carbonyl-based processes to include the synthesis of acrylic acid, acetic acid, and other valuable chemicals.
In the post-war era of the 1950s and 1960s, carbonyl processes gained significant industrial traction. The Monsanto acetic acid process, developed in the 1960s, revolutionized the production of acetic acid using a rhodium-based catalyst. This period also witnessed the emergence of hydroformylation as a major industrial process for the production of aldehydes and alcohols.
The 1970s and 1980s marked a shift towards more efficient and selective catalysts. The development of ligand-modified rhodium catalysts by Union Carbide significantly improved the regioselectivity of hydroformylation reactions. Concurrently, advances in process engineering led to more efficient reactor designs and separation techniques.
The late 20th century saw a growing emphasis on environmental considerations. This led to the development of greener carbonyl processes, including the use of supercritical CO2 as a solvent and the exploration of biocatalytic routes. The turn of the millennium brought increased focus on atom economy and waste reduction, driving research into tandem and cascade reactions involving carbonyl chemistry.
In recent years, the evolution of carbonyl processes has been characterized by a push towards sustainability. This has manifested in several ways: the use of renewable feedstocks, the development of catalysts based on earth-abundant metals, and the integration of carbonyl chemistry with other green technologies such as electrochemistry and photochemistry.
The advent of high-throughput experimentation and computational modeling has accelerated the pace of innovation in carbonyl chemistry. These tools have enabled rapid screening of catalysts and reaction conditions, leading to more efficient and selective processes. Additionally, the application of flow chemistry to carbonyl processes has opened new avenues for process intensification and continuous manufacturing.
Looking forward, the evolution of carbonyl-based processes is likely to continue along the path of sustainability and efficiency. Emerging areas of research include the development of bio-based platform chemicals through carbonyl chemistry, the use of CO2 as a C1 building block, and the integration of carbonyl processes with renewable energy sources. These developments promise to further enhance the role of carbonyl chemistry in driving sustainable production in the chemical industry.
The foundation of carbonyl chemistry was laid in the 1920s with the discovery of the oxo synthesis by Otto Roelen. This breakthrough paved the way for the industrial production of aldehydes and alcohols from olefins and syngas. The 1930s and 1940s saw the development of the Reppe chemistry, which expanded the scope of carbonyl-based processes to include the synthesis of acrylic acid, acetic acid, and other valuable chemicals.
In the post-war era of the 1950s and 1960s, carbonyl processes gained significant industrial traction. The Monsanto acetic acid process, developed in the 1960s, revolutionized the production of acetic acid using a rhodium-based catalyst. This period also witnessed the emergence of hydroformylation as a major industrial process for the production of aldehydes and alcohols.
The 1970s and 1980s marked a shift towards more efficient and selective catalysts. The development of ligand-modified rhodium catalysts by Union Carbide significantly improved the regioselectivity of hydroformylation reactions. Concurrently, advances in process engineering led to more efficient reactor designs and separation techniques.
The late 20th century saw a growing emphasis on environmental considerations. This led to the development of greener carbonyl processes, including the use of supercritical CO2 as a solvent and the exploration of biocatalytic routes. The turn of the millennium brought increased focus on atom economy and waste reduction, driving research into tandem and cascade reactions involving carbonyl chemistry.
In recent years, the evolution of carbonyl processes has been characterized by a push towards sustainability. This has manifested in several ways: the use of renewable feedstocks, the development of catalysts based on earth-abundant metals, and the integration of carbonyl chemistry with other green technologies such as electrochemistry and photochemistry.
The advent of high-throughput experimentation and computational modeling has accelerated the pace of innovation in carbonyl chemistry. These tools have enabled rapid screening of catalysts and reaction conditions, leading to more efficient and selective processes. Additionally, the application of flow chemistry to carbonyl processes has opened new avenues for process intensification and continuous manufacturing.
Looking forward, the evolution of carbonyl-based processes is likely to continue along the path of sustainability and efficiency. Emerging areas of research include the development of bio-based platform chemicals through carbonyl chemistry, the use of CO2 as a C1 building block, and the integration of carbonyl processes with renewable energy sources. These developments promise to further enhance the role of carbonyl chemistry in driving sustainable production in the chemical industry.
Sustainable Production Demand
The demand for sustainable production processes has become increasingly urgent in recent years, driven by growing environmental concerns, regulatory pressures, and consumer preferences. Carbonyl-based processes have emerged as a promising avenue for achieving sustainability in various industrial sectors, particularly in chemical manufacturing, pharmaceuticals, and materials production.
Market analysis indicates a significant shift towards eco-friendly production methods, with the global green chemicals market expected to reach substantial growth in the coming years. This trend is fueled by stringent environmental regulations, such as the European Union's Green Deal and similar initiatives worldwide, which aim to reduce carbon emissions and promote circular economy principles.
In the chemical industry, there is a growing demand for sustainable alternatives to traditional petrochemical-based processes. Carbonyl-based reactions offer potential solutions for synthesizing valuable compounds using renewable feedstocks and more environmentally benign conditions. This aligns with the industry's push towards green chemistry principles and the development of bio-based chemicals.
The pharmaceutical sector is also experiencing increased pressure to adopt more sustainable production methods. Carbonyl chemistry plays a crucial role in drug synthesis, and there is a rising interest in developing greener approaches to these reactions. This includes the use of biocatalysts, continuous flow processes, and alternative solvents, all of which can contribute to reducing the environmental footprint of pharmaceutical manufacturing.
In materials production, carbonyl-based processes are gaining attention for their potential in creating sustainable polymers and advanced materials. There is a growing market demand for biodegradable plastics and bio-based materials, driven by concerns over plastic pollution and the need for more sustainable packaging solutions.
The adoption of carbonyl-based sustainable production processes is also influenced by the increasing focus on lifecycle assessment and circular economy principles. Companies are seeking ways to reduce waste, improve resource efficiency, and minimize the overall environmental impact of their production processes. This has led to a rising interest in technologies that can enable the valorization of waste streams and the development of closed-loop production systems.
Furthermore, there is a notable trend towards the integration of renewable energy sources in chemical production processes. This synergy between sustainable energy and carbonyl-based chemistry presents opportunities for developing innovative, carbon-neutral production methods that can meet the growing demand for environmentally responsible manufacturing practices.
Market analysis indicates a significant shift towards eco-friendly production methods, with the global green chemicals market expected to reach substantial growth in the coming years. This trend is fueled by stringent environmental regulations, such as the European Union's Green Deal and similar initiatives worldwide, which aim to reduce carbon emissions and promote circular economy principles.
In the chemical industry, there is a growing demand for sustainable alternatives to traditional petrochemical-based processes. Carbonyl-based reactions offer potential solutions for synthesizing valuable compounds using renewable feedstocks and more environmentally benign conditions. This aligns with the industry's push towards green chemistry principles and the development of bio-based chemicals.
The pharmaceutical sector is also experiencing increased pressure to adopt more sustainable production methods. Carbonyl chemistry plays a crucial role in drug synthesis, and there is a rising interest in developing greener approaches to these reactions. This includes the use of biocatalysts, continuous flow processes, and alternative solvents, all of which can contribute to reducing the environmental footprint of pharmaceutical manufacturing.
In materials production, carbonyl-based processes are gaining attention for their potential in creating sustainable polymers and advanced materials. There is a growing market demand for biodegradable plastics and bio-based materials, driven by concerns over plastic pollution and the need for more sustainable packaging solutions.
The adoption of carbonyl-based sustainable production processes is also influenced by the increasing focus on lifecycle assessment and circular economy principles. Companies are seeking ways to reduce waste, improve resource efficiency, and minimize the overall environmental impact of their production processes. This has led to a rising interest in technologies that can enable the valorization of waste streams and the development of closed-loop production systems.
Furthermore, there is a notable trend towards the integration of renewable energy sources in chemical production processes. This synergy between sustainable energy and carbonyl-based chemistry presents opportunities for developing innovative, carbon-neutral production methods that can meet the growing demand for environmentally responsible manufacturing practices.
Carbonyl Tech Challenges
Carbonyl-based processes have emerged as a promising avenue for sustainable production, yet they face several significant challenges that hinder their widespread adoption and efficiency. One of the primary obstacles is the high energy consumption associated with these processes. Traditional carbonyl reactions often require elevated temperatures and pressures, leading to substantial energy inputs and increased operational costs. This energy-intensive nature not only impacts the economic viability of carbonyl-based production but also contradicts the sustainability goals these processes aim to achieve.
Another critical challenge lies in the selectivity and yield of carbonyl reactions. Many carbonyl-based processes suffer from poor selectivity, resulting in the formation of unwanted by-products. This not only reduces the overall efficiency of the process but also necessitates additional purification steps, further increasing energy consumption and waste generation. Improving reaction selectivity while maintaining high yields remains a significant hurdle for researchers and industry professionals alike.
The stability and reactivity of carbonyl compounds pose additional challenges. Some carbonyl intermediates are highly reactive and prone to side reactions or decomposition, making them difficult to handle and control in industrial settings. This instability can lead to safety concerns, reduced product quality, and increased process complexity. Developing strategies to stabilize reactive carbonyl species or design more robust reaction pathways is crucial for advancing carbonyl-based sustainable production.
Catalyst design and optimization represent another major challenge in this field. While catalysts play a vital role in enhancing reaction rates and selectivity, finding catalysts that are both highly active and stable under the reaction conditions of carbonyl processes can be challenging. Many existing catalysts suffer from deactivation or leaching issues, limiting their long-term effectiveness and recyclability. The development of novel, efficient, and durable catalysts tailored for carbonyl-based reactions is essential for improving process sustainability and economic viability.
Furthermore, the scalability of carbonyl-based processes presents significant engineering challenges. Many promising reactions demonstrated at the laboratory scale face difficulties when scaled up to industrial production levels. Issues such as heat and mass transfer limitations, reactor design complexities, and process control become more pronounced at larger scales. Overcoming these scaling challenges is crucial for the successful implementation of carbonyl-based sustainable production technologies in real-world applications.
Lastly, the environmental impact of carbonyl-based processes, particularly in terms of waste generation and potential emissions, remains a concern. While these processes aim to enhance sustainability, they must be carefully evaluated and optimized to minimize their environmental footprint. This includes addressing issues such as solvent use, waste treatment, and the potential release of volatile organic compounds. Developing greener reaction conditions and more environmentally benign methodologies is essential for truly realizing the sustainable potential of carbonyl-based production processes.
Another critical challenge lies in the selectivity and yield of carbonyl reactions. Many carbonyl-based processes suffer from poor selectivity, resulting in the formation of unwanted by-products. This not only reduces the overall efficiency of the process but also necessitates additional purification steps, further increasing energy consumption and waste generation. Improving reaction selectivity while maintaining high yields remains a significant hurdle for researchers and industry professionals alike.
The stability and reactivity of carbonyl compounds pose additional challenges. Some carbonyl intermediates are highly reactive and prone to side reactions or decomposition, making them difficult to handle and control in industrial settings. This instability can lead to safety concerns, reduced product quality, and increased process complexity. Developing strategies to stabilize reactive carbonyl species or design more robust reaction pathways is crucial for advancing carbonyl-based sustainable production.
Catalyst design and optimization represent another major challenge in this field. While catalysts play a vital role in enhancing reaction rates and selectivity, finding catalysts that are both highly active and stable under the reaction conditions of carbonyl processes can be challenging. Many existing catalysts suffer from deactivation or leaching issues, limiting their long-term effectiveness and recyclability. The development of novel, efficient, and durable catalysts tailored for carbonyl-based reactions is essential for improving process sustainability and economic viability.
Furthermore, the scalability of carbonyl-based processes presents significant engineering challenges. Many promising reactions demonstrated at the laboratory scale face difficulties when scaled up to industrial production levels. Issues such as heat and mass transfer limitations, reactor design complexities, and process control become more pronounced at larger scales. Overcoming these scaling challenges is crucial for the successful implementation of carbonyl-based sustainable production technologies in real-world applications.
Lastly, the environmental impact of carbonyl-based processes, particularly in terms of waste generation and potential emissions, remains a concern. While these processes aim to enhance sustainability, they must be carefully evaluated and optimized to minimize their environmental footprint. This includes addressing issues such as solvent use, waste treatment, and the potential release of volatile organic compounds. Developing greener reaction conditions and more environmentally benign methodologies is essential for truly realizing the sustainable potential of carbonyl-based production processes.
Current Carbonyl Solutions
01 Sustainable carbonyl compound production
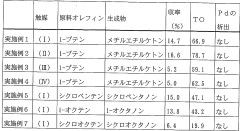
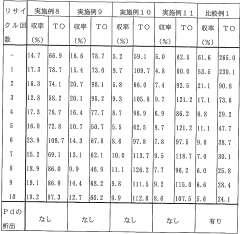
Processes for producing carbonyl compounds using sustainable methods, focusing on environmentally friendly catalysts and renewable feedstocks. These methods aim to reduce the carbon footprint of carbonyl compound production while maintaining or improving efficiency.- Sustainable carbonyl compound production: Processes for sustainable production of carbonyl compounds using environmentally friendly methods and renewable resources. These methods aim to reduce environmental impact and improve overall process efficiency in the synthesis of various carbonyl-containing products.
- Catalytic processes for carbonyl synthesis: Development of novel catalytic systems for the efficient and selective production of carbonyl compounds. These catalysts enable more sustainable reaction pathways, lower energy requirements, and improved yields in carbonyl-based processes.
- Biomass-derived carbonyl compounds: Utilization of biomass feedstocks for the production of carbonyl compounds, offering a renewable alternative to traditional petrochemical-based processes. These methods focus on converting biomass into valuable carbonyl-containing chemicals and materials.
- Green solvents in carbonyl processes: Implementation of green solvents and solvent-free approaches in carbonyl-based reactions to enhance sustainability. These methods reduce the environmental impact of solvent use and improve the overall efficiency of carbonyl compound production.
- Continuous flow processes for carbonyl synthesis: Development of continuous flow technologies for the sustainable production of carbonyl compounds. These processes offer advantages such as improved heat and mass transfer, reduced reaction times, and enhanced control over reaction parameters, leading to more efficient and environmentally friendly carbonyl synthesis.
02 Biomass-derived carbonyl compounds
Techniques for converting biomass into valuable carbonyl compounds, utilizing various catalytic processes and reaction conditions. These methods offer a renewable alternative to traditional petrochemical-based production of carbonyl compounds.Expand Specific Solutions03 Electrochemical carbonyl synthesis
Electrochemical methods for producing carbonyl compounds, employing sustainable electricity sources and optimized electrode materials. These processes aim to reduce energy consumption and improve selectivity in carbonyl compound synthesis.Expand Specific Solutions04 Continuous flow carbonyl processes
Development of continuous flow reactors and processes for sustainable carbonyl compound production. These systems offer improved efficiency, reduced waste, and better control over reaction parameters compared to batch processes.Expand Specific Solutions05 Green solvents in carbonyl synthesis
Incorporation of green solvents, such as ionic liquids or supercritical fluids, in carbonyl compound production processes. These alternative solvents aim to reduce environmental impact and improve overall sustainability of the synthesis.Expand Specific Solutions
Carbonyl Tech Innovations
Method for producing carbonyl compound
PatentWO2019172360A1
Innovation
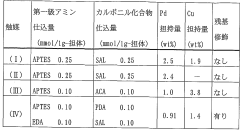
- A method involving an olefin compound, a non-alcoholic organic solvent, water, a metal catalyst, and an oxidizing agent to produce carbonyl compounds, specifically using a Pd catalyst and molecular oxygen, which enhances the reaction efficiency and selectivity.
Process for producing carbonyl compound
PatentWO2004048306A1
Innovation
- A method involving a catalyst system comprising a palladium compound, a heteropolyacid, and an imine compound, where the palladium and imine are immobilized on a carrier, suppressing palladium deposition and enabling stable, high-reactivity carbonyl compound production.
Green Chemistry Regulations
Green chemistry regulations play a crucial role in shaping the landscape of sustainable production processes, particularly in the context of carbonyl-based chemistry. These regulations aim to minimize environmental impact, reduce waste generation, and promote the use of safer chemicals and processes throughout the industry.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation stands as a cornerstone of green chemistry legislation. It requires companies to register chemical substances and demonstrate their safe use, encouraging the development of more environmentally friendly alternatives. This has significantly influenced carbonyl-based processes, pushing manufacturers to explore greener synthesis routes and catalysts.
In the United States, the Pollution Prevention Act and the Toxic Substances Control Act (TSCA) have been instrumental in promoting green chemistry principles. These regulations emphasize the importance of preventing pollution at the source rather than treating it after generation. As a result, industries involved in carbonyl chemistry have been incentivized to develop cleaner production methods and adopt more sustainable practices.
The concept of "design for degradation" has gained traction in green chemistry regulations worldwide. This principle encourages the development of chemicals and materials that can break down into harmless substances after use. For carbonyl-based processes, this has led to increased research into biodegradable alternatives and the exploration of bio-based feedstocks as starting materials.
Green chemistry metrics, such as the E-factor (Environmental factor) and atom economy, have been incorporated into regulatory frameworks to assess the environmental impact of chemical processes. These metrics help quantify the efficiency and sustainability of carbonyl-based reactions, guiding researchers and manufacturers towards more environmentally friendly methodologies.
Regulations promoting the use of renewable resources have also impacted carbonyl chemistry. Many countries have implemented policies encouraging the utilization of biomass-derived starting materials, leading to the development of novel carbonyl compounds from renewable sources. This shift aligns with the broader goal of reducing dependence on fossil fuel-based feedstocks.
The implementation of life cycle assessment (LCA) requirements in green chemistry regulations has forced industries to consider the entire environmental footprint of their processes. This holistic approach has led to innovations in carbonyl chemistry, such as the development of more energy-efficient synthesis routes and the exploration of alternative solvents with lower environmental impact.
As green chemistry regulations continue to evolve, they are expected to drive further advancements in sustainable carbonyl-based processes. Future regulatory trends may include stricter limits on volatile organic compound (VOC) emissions, increased emphasis on circular economy principles, and the promotion of bio-based alternatives to traditional petrochemical-derived carbonyl compounds.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation stands as a cornerstone of green chemistry legislation. It requires companies to register chemical substances and demonstrate their safe use, encouraging the development of more environmentally friendly alternatives. This has significantly influenced carbonyl-based processes, pushing manufacturers to explore greener synthesis routes and catalysts.
In the United States, the Pollution Prevention Act and the Toxic Substances Control Act (TSCA) have been instrumental in promoting green chemistry principles. These regulations emphasize the importance of preventing pollution at the source rather than treating it after generation. As a result, industries involved in carbonyl chemistry have been incentivized to develop cleaner production methods and adopt more sustainable practices.
The concept of "design for degradation" has gained traction in green chemistry regulations worldwide. This principle encourages the development of chemicals and materials that can break down into harmless substances after use. For carbonyl-based processes, this has led to increased research into biodegradable alternatives and the exploration of bio-based feedstocks as starting materials.
Green chemistry metrics, such as the E-factor (Environmental factor) and atom economy, have been incorporated into regulatory frameworks to assess the environmental impact of chemical processes. These metrics help quantify the efficiency and sustainability of carbonyl-based reactions, guiding researchers and manufacturers towards more environmentally friendly methodologies.
Regulations promoting the use of renewable resources have also impacted carbonyl chemistry. Many countries have implemented policies encouraging the utilization of biomass-derived starting materials, leading to the development of novel carbonyl compounds from renewable sources. This shift aligns with the broader goal of reducing dependence on fossil fuel-based feedstocks.
The implementation of life cycle assessment (LCA) requirements in green chemistry regulations has forced industries to consider the entire environmental footprint of their processes. This holistic approach has led to innovations in carbonyl chemistry, such as the development of more energy-efficient synthesis routes and the exploration of alternative solvents with lower environmental impact.
As green chemistry regulations continue to evolve, they are expected to drive further advancements in sustainable carbonyl-based processes. Future regulatory trends may include stricter limits on volatile organic compound (VOC) emissions, increased emphasis on circular economy principles, and the promotion of bio-based alternatives to traditional petrochemical-derived carbonyl compounds.
Life Cycle Assessment
Life Cycle Assessment (LCA) plays a crucial role in evaluating the environmental impacts of carbonyl-based processes driving sustainable production. This comprehensive approach examines the entire lifecycle of products and processes, from raw material extraction to end-of-life disposal, providing valuable insights into their sustainability performance.
In the context of carbonyl-based processes, LCA helps identify hotspots of environmental impact throughout the production chain. These assessments typically consider multiple impact categories, including global warming potential, energy consumption, water usage, and resource depletion. By quantifying these impacts, researchers and industry professionals can make informed decisions to optimize process efficiency and reduce environmental footprints.
One key aspect of LCA in carbonyl-based processes is the evaluation of feedstock sources. Comparing fossil-based and bio-based raw materials allows for a thorough understanding of the environmental trade-offs associated with different feedstock options. This analysis often reveals unexpected insights, such as the potential for increased land use or water consumption in bio-based processes, despite their lower carbon footprint.
Energy consumption is another critical factor in LCA studies of carbonyl-based processes. These assessments help identify energy-intensive steps in the production chain, enabling targeted improvements in energy efficiency. Furthermore, LCA can evaluate the potential benefits of integrating renewable energy sources into the production process, contributing to overall sustainability goals.
The end-of-life phase of carbonyl-based products is also a significant consideration in LCA. Assessing various disposal scenarios, including recycling, incineration, and landfilling, provides valuable information on the long-term environmental impacts of these products. This analysis can drive innovations in product design and recycling technologies to minimize waste and promote circular economy principles.
LCA results often inform decision-making processes in industry and policy-making. By comparing different production routes and technologies, stakeholders can identify the most environmentally friendly options for carbonyl-based processes. This information is invaluable for guiding research and development efforts towards more sustainable production methods.
However, it is important to note that LCA studies in this field face certain challenges. The complexity of carbonyl-based processes and the variability in production methods can lead to uncertainties in LCA results. Additionally, the availability and quality of data can significantly impact the accuracy of these assessments. Ongoing research aims to address these challenges and improve the reliability of LCA in the context of sustainable carbonyl-based production.
In the context of carbonyl-based processes, LCA helps identify hotspots of environmental impact throughout the production chain. These assessments typically consider multiple impact categories, including global warming potential, energy consumption, water usage, and resource depletion. By quantifying these impacts, researchers and industry professionals can make informed decisions to optimize process efficiency and reduce environmental footprints.
One key aspect of LCA in carbonyl-based processes is the evaluation of feedstock sources. Comparing fossil-based and bio-based raw materials allows for a thorough understanding of the environmental trade-offs associated with different feedstock options. This analysis often reveals unexpected insights, such as the potential for increased land use or water consumption in bio-based processes, despite their lower carbon footprint.
Energy consumption is another critical factor in LCA studies of carbonyl-based processes. These assessments help identify energy-intensive steps in the production chain, enabling targeted improvements in energy efficiency. Furthermore, LCA can evaluate the potential benefits of integrating renewable energy sources into the production process, contributing to overall sustainability goals.
The end-of-life phase of carbonyl-based products is also a significant consideration in LCA. Assessing various disposal scenarios, including recycling, incineration, and landfilling, provides valuable information on the long-term environmental impacts of these products. This analysis can drive innovations in product design and recycling technologies to minimize waste and promote circular economy principles.
LCA results often inform decision-making processes in industry and policy-making. By comparing different production routes and technologies, stakeholders can identify the most environmentally friendly options for carbonyl-based processes. This information is invaluable for guiding research and development efforts towards more sustainable production methods.
However, it is important to note that LCA studies in this field face certain challenges. The complexity of carbonyl-based processes and the variability in production methods can lead to uncertainties in LCA results. Additionally, the availability and quality of data can significantly impact the accuracy of these assessments. Ongoing research aims to address these challenges and improve the reliability of LCA in the context of sustainable carbonyl-based production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!