Carbonyl Chemistry Contributions to Material Resilience
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Chemistry Evolution and Objectives
Carbonyl chemistry has played a pivotal role in the development of resilient materials over the past century. The evolution of this field has been marked by significant breakthroughs in understanding the reactivity and versatility of carbonyl compounds. From early discoveries in organic synthesis to modern applications in polymer science, carbonyl chemistry has continuously expanded its scope and impact on material science.
The primary objective in this field has been to harness the unique properties of carbonyl groups to enhance material resilience. This includes improving mechanical strength, thermal stability, and chemical resistance of various materials. Researchers have focused on exploiting the reactivity of carbonyl compounds to create cross-linked networks, self-healing polymers, and advanced composite materials.
One of the key trends in carbonyl chemistry has been the development of reversible reactions. These reactions allow for the creation of dynamic covalent networks, which can adapt to environmental stresses and recover from damage. This approach has opened new avenues for designing smart materials with self-healing capabilities and enhanced durability.
Another significant trend is the integration of carbonyl chemistry with sustainable practices. Scientists are increasingly exploring bio-based carbonyl compounds and environmentally friendly synthesis methods. This shift aligns with the growing demand for sustainable materials and processes in various industries.
The evolution of analytical techniques has greatly contributed to advancements in carbonyl chemistry. Advanced spectroscopic methods and computational modeling have enabled researchers to gain deeper insights into reaction mechanisms and molecular interactions. This has led to more precise control over material properties and the ability to tailor materials for specific applications.
Looking forward, the objectives in carbonyl chemistry for material resilience are multifaceted. Researchers aim to develop materials with unprecedented levels of toughness, self-repair capabilities, and adaptability to extreme conditions. There is also a strong focus on creating multi-functional materials that can respond to various stimuli, such as temperature, light, or mechanical stress.
Another key objective is to bridge the gap between laboratory discoveries and industrial applications. This involves scaling up production processes, optimizing material performance, and ensuring cost-effectiveness. Researchers are also working on integrating carbonyl chemistry with other emerging technologies, such as nanotechnology and 3D printing, to create novel materials with enhanced resilience and functionality.
The primary objective in this field has been to harness the unique properties of carbonyl groups to enhance material resilience. This includes improving mechanical strength, thermal stability, and chemical resistance of various materials. Researchers have focused on exploiting the reactivity of carbonyl compounds to create cross-linked networks, self-healing polymers, and advanced composite materials.
One of the key trends in carbonyl chemistry has been the development of reversible reactions. These reactions allow for the creation of dynamic covalent networks, which can adapt to environmental stresses and recover from damage. This approach has opened new avenues for designing smart materials with self-healing capabilities and enhanced durability.
Another significant trend is the integration of carbonyl chemistry with sustainable practices. Scientists are increasingly exploring bio-based carbonyl compounds and environmentally friendly synthesis methods. This shift aligns with the growing demand for sustainable materials and processes in various industries.
The evolution of analytical techniques has greatly contributed to advancements in carbonyl chemistry. Advanced spectroscopic methods and computational modeling have enabled researchers to gain deeper insights into reaction mechanisms and molecular interactions. This has led to more precise control over material properties and the ability to tailor materials for specific applications.
Looking forward, the objectives in carbonyl chemistry for material resilience are multifaceted. Researchers aim to develop materials with unprecedented levels of toughness, self-repair capabilities, and adaptability to extreme conditions. There is also a strong focus on creating multi-functional materials that can respond to various stimuli, such as temperature, light, or mechanical stress.
Another key objective is to bridge the gap between laboratory discoveries and industrial applications. This involves scaling up production processes, optimizing material performance, and ensuring cost-effectiveness. Researchers are also working on integrating carbonyl chemistry with other emerging technologies, such as nanotechnology and 3D printing, to create novel materials with enhanced resilience and functionality.
Market Demand for Resilient Materials
The market demand for resilient materials has been steadily increasing across various industries, driven by the need for durable and long-lasting products in challenging environments. Carbonyl chemistry plays a crucial role in enhancing material resilience, making it a key focus area for research and development in the materials science sector.
In the automotive industry, there is a growing demand for resilient materials that can withstand extreme temperatures, chemical exposure, and mechanical stress. Carbonyl-based polymers and composites are being sought after for their ability to improve the longevity and performance of vehicle components, particularly in engine parts and exterior coatings.
The construction sector is another major driver of demand for resilient materials. With the increasing focus on sustainable and long-lasting infrastructure, there is a significant market for materials that can resist weathering, corrosion, and structural degradation. Carbonyl-modified concrete additives and protective coatings are gaining traction in this sector, offering enhanced durability and reduced maintenance costs.
In the aerospace industry, the demand for lightweight yet resilient materials is paramount. Carbonyl-based composites are being explored for their potential to create aircraft components that can withstand high-stress environments while maintaining structural integrity over extended periods. This market segment is expected to see substantial growth as manufacturers seek to improve fuel efficiency and reduce maintenance intervals.
The electronics industry is also contributing to the market demand for resilient materials. As consumer electronics become more integrated into daily life, there is an increasing need for devices that can withstand accidental drops, water exposure, and temperature fluctuations. Carbonyl chemistry is being leveraged to develop protective coatings and encapsulation materials that enhance the durability of electronic components.
Environmental concerns are further driving the demand for resilient materials in the packaging industry. There is a growing market for biodegradable yet durable packaging solutions that can protect products throughout the supply chain while minimizing environmental impact. Carbonyl-based biopolymers are being developed to meet this demand, offering a balance between resilience and sustainability.
The medical device industry represents another significant market for resilient materials. With the increasing complexity of implantable devices and the need for biocompatibility, there is a demand for materials that can maintain their properties in the human body over extended periods. Carbonyl-modified polymers are being investigated for their potential to create long-lasting and biocompatible medical implants and devices.
As industries continue to push the boundaries of material performance, the market for resilient materials enhanced by carbonyl chemistry is expected to expand. This growth is likely to be accompanied by increased research and development efforts, as well as collaborations between material scientists, chemists, and industry partners to develop innovative solutions that meet the evolving demands of various sectors.
In the automotive industry, there is a growing demand for resilient materials that can withstand extreme temperatures, chemical exposure, and mechanical stress. Carbonyl-based polymers and composites are being sought after for their ability to improve the longevity and performance of vehicle components, particularly in engine parts and exterior coatings.
The construction sector is another major driver of demand for resilient materials. With the increasing focus on sustainable and long-lasting infrastructure, there is a significant market for materials that can resist weathering, corrosion, and structural degradation. Carbonyl-modified concrete additives and protective coatings are gaining traction in this sector, offering enhanced durability and reduced maintenance costs.
In the aerospace industry, the demand for lightweight yet resilient materials is paramount. Carbonyl-based composites are being explored for their potential to create aircraft components that can withstand high-stress environments while maintaining structural integrity over extended periods. This market segment is expected to see substantial growth as manufacturers seek to improve fuel efficiency and reduce maintenance intervals.
The electronics industry is also contributing to the market demand for resilient materials. As consumer electronics become more integrated into daily life, there is an increasing need for devices that can withstand accidental drops, water exposure, and temperature fluctuations. Carbonyl chemistry is being leveraged to develop protective coatings and encapsulation materials that enhance the durability of electronic components.
Environmental concerns are further driving the demand for resilient materials in the packaging industry. There is a growing market for biodegradable yet durable packaging solutions that can protect products throughout the supply chain while minimizing environmental impact. Carbonyl-based biopolymers are being developed to meet this demand, offering a balance between resilience and sustainability.
The medical device industry represents another significant market for resilient materials. With the increasing complexity of implantable devices and the need for biocompatibility, there is a demand for materials that can maintain their properties in the human body over extended periods. Carbonyl-modified polymers are being investigated for their potential to create long-lasting and biocompatible medical implants and devices.
As industries continue to push the boundaries of material performance, the market for resilient materials enhanced by carbonyl chemistry is expected to expand. This growth is likely to be accompanied by increased research and development efforts, as well as collaborations between material scientists, chemists, and industry partners to develop innovative solutions that meet the evolving demands of various sectors.
Current Challenges in Carbonyl-based Material Resilience
The field of carbonyl-based material resilience faces several significant challenges that hinder its widespread application and advancement. One of the primary obstacles is the inherent reactivity of carbonyl groups, which can lead to undesired side reactions and degradation of materials over time. This reactivity, while beneficial in some applications, often compromises the long-term stability and performance of carbonyl-containing materials in harsh environments or under prolonged stress.
Another major challenge lies in controlling the crosslinking density and distribution in carbonyl-based polymers and composites. Achieving the optimal balance between flexibility and strength remains a complex task, as excessive crosslinking can result in brittle materials, while insufficient crosslinking may lead to poor mechanical properties and reduced resilience.
The sensitivity of carbonyl compounds to moisture and environmental factors poses additional difficulties in maintaining material integrity. Hydrolysis and oxidation reactions can significantly alter the structure and properties of carbonyl-based materials, necessitating the development of effective protection strategies and stabilization techniques.
Furthermore, the scalability of carbonyl chemistry in material production presents challenges in terms of cost-effectiveness and process control. Ensuring consistent quality and properties across large-scale manufacturing processes remains a hurdle for many industries seeking to implement carbonyl-based resilient materials.
The environmental impact of carbonyl chemistry in material production is also a growing concern. Many traditional synthesis methods involve the use of toxic reagents or generate harmful byproducts, prompting the need for greener and more sustainable approaches to carbonyl-based material development.
Lastly, the integration of carbonyl-based materials with other components in complex systems presents compatibility issues. Interactions between carbonyl groups and other functional moieties can lead to unexpected changes in material properties or performance, requiring careful consideration in material design and application.
Addressing these challenges requires interdisciplinary efforts combining organic chemistry, materials science, and engineering. Innovations in synthetic methodologies, protective strategies, and characterization techniques are essential to overcome the current limitations and unlock the full potential of carbonyl chemistry in enhancing material resilience.
Another major challenge lies in controlling the crosslinking density and distribution in carbonyl-based polymers and composites. Achieving the optimal balance between flexibility and strength remains a complex task, as excessive crosslinking can result in brittle materials, while insufficient crosslinking may lead to poor mechanical properties and reduced resilience.
The sensitivity of carbonyl compounds to moisture and environmental factors poses additional difficulties in maintaining material integrity. Hydrolysis and oxidation reactions can significantly alter the structure and properties of carbonyl-based materials, necessitating the development of effective protection strategies and stabilization techniques.
Furthermore, the scalability of carbonyl chemistry in material production presents challenges in terms of cost-effectiveness and process control. Ensuring consistent quality and properties across large-scale manufacturing processes remains a hurdle for many industries seeking to implement carbonyl-based resilient materials.
The environmental impact of carbonyl chemistry in material production is also a growing concern. Many traditional synthesis methods involve the use of toxic reagents or generate harmful byproducts, prompting the need for greener and more sustainable approaches to carbonyl-based material development.
Lastly, the integration of carbonyl-based materials with other components in complex systems presents compatibility issues. Interactions between carbonyl groups and other functional moieties can lead to unexpected changes in material properties or performance, requiring careful consideration in material design and application.
Addressing these challenges requires interdisciplinary efforts combining organic chemistry, materials science, and engineering. Innovations in synthetic methodologies, protective strategies, and characterization techniques are essential to overcome the current limitations and unlock the full potential of carbonyl chemistry in enhancing material resilience.
Existing Carbonyl Chemistry Solutions for Material Resilience
01 Carbonyl-containing polymers for enhanced material resilience
Carbonyl-containing polymers are utilized to improve the resilience of materials. These polymers can be incorporated into various substrates to enhance their mechanical properties, including elasticity and durability. The carbonyl groups in the polymer structure contribute to the formation of intermolecular bonds, resulting in improved material strength and resistance to deformation.- Carbonyl-containing polymers for enhanced material resilience: Incorporation of carbonyl groups into polymer structures can significantly improve material resilience. These carbonyl-containing polymers exhibit enhanced mechanical properties, including improved tensile strength and elasticity. The presence of carbonyl groups allows for better intermolecular interactions, leading to increased durability and resistance to environmental stressors.
- Cross-linking mechanisms utilizing carbonyl chemistry: Cross-linking reactions involving carbonyl groups can be employed to enhance material resilience. These mechanisms often involve the formation of covalent bonds between polymer chains, resulting in improved mechanical properties and chemical resistance. Various cross-linking agents and catalysts can be used to optimize the process for specific applications.
- Carbonyl-based coatings for surface protection: Coatings containing carbonyl compounds can be applied to surfaces to enhance their resilience. These coatings provide protection against environmental factors such as UV radiation, moisture, and chemical exposure. The carbonyl groups in the coating formulations contribute to improved adhesion and durability of the protective layer.
- Carbonyl chemistry in self-healing materials: Self-healing materials incorporating carbonyl chemistry have been developed to enhance material resilience. These materials can autonomously repair damage through reversible bond formation involving carbonyl groups. This self-healing capability extends the lifespan of materials and improves their overall durability in various applications.
- Nanocomposites with carbonyl-functionalized fillers: Nanocomposites incorporating carbonyl-functionalized fillers exhibit enhanced material resilience. The carbonyl groups on the filler surfaces improve interfacial interactions with the polymer matrix, leading to better dispersion and stronger reinforcement effects. These nanocomposites demonstrate improved mechanical properties and resistance to environmental degradation.
02 Crosslinking agents with carbonyl functionality for material reinforcement
Crosslinking agents containing carbonyl groups are employed to reinforce materials and enhance their resilience. These agents form covalent bonds between polymer chains, creating a more robust network structure. The resulting materials exhibit improved mechanical properties, including increased tensile strength, tear resistance, and overall durability.Expand Specific Solutions03 Carbonyl-based coatings for surface protection and resilience
Coatings formulated with carbonyl-containing compounds are applied to surfaces to enhance their resilience and protective properties. These coatings form a durable layer that resists wear, abrasion, and environmental factors. The carbonyl groups in the coating formulation contribute to improved adhesion and chemical resistance, resulting in longer-lasting and more resilient surfaces.Expand Specific Solutions04 Carbonyl chemistry in self-healing materials
Carbonyl chemistry plays a crucial role in the development of self-healing materials with enhanced resilience. These materials incorporate carbonyl-containing compounds that can undergo reversible reactions, allowing the material to repair damage autonomously. This self-healing capability contributes to improved longevity and sustained performance of the material under various stress conditions.Expand Specific Solutions05 Carbonyl-functionalized nanocomposites for improved material properties
Nanocomposites incorporating carbonyl-functionalized nanoparticles or nanostructures are developed to enhance material resilience. The carbonyl groups on the surface of these nanostructures facilitate better interaction with the matrix material, resulting in improved mechanical properties, thermal stability, and overall durability of the composite. These nanocomposites find applications in various fields where high-performance materials are required.Expand Specific Solutions
Key Players in Carbonyl-based Material Industry
The competitive landscape for "Carbonyl Chemistry Contributions to Material Resilience" is in a growth phase, with increasing market size and technological advancements. The industry is characterized by a mix of established chemical companies and research institutions driving innovation. Key players like Sekisui Chemical, BASF, and Solvay are leveraging their expertise in specialty chemicals to develop resilient materials. Universities and research labs, such as East China University of Science & Technology and the Naval Research Laboratory, are contributing to fundamental research. The technology is maturing, with companies like Toray Industries and PPG Industries applying carbonyl chemistry principles to enhance material properties for various industrial applications.
Sekisui Chemical Co., Ltd.
Technical Solution: Sekisui Chemical has leveraged carbonyl chemistry to enhance the resilience of their building and infrastructure materials. They have developed carbonyl-modified polyvinyl chloride (PVC) formulations that show improved weatherability and UV resistance for long-lasting exterior applications[9]. Their research into carbonyl-containing intumescent coatings has resulted in advanced fire-resistant materials for construction[10]. Sekisui has also created carbonyl-functionalized polyolefins with improved adhesion properties, enhancing the durability of multi-layer pipes and films used in infrastructure projects[11].
Strengths: Strong position in housing and infrastructure markets, focus on environmentally friendly products. Weaknesses: Relatively limited global presence compared to some competitors, potential regulatory challenges in certain markets.
BASF Corp.
Technical Solution: BASF has developed advanced carbonyl-based polymers for enhanced material resilience. Their approach involves incorporating carbonyl groups into polymer chains to improve oxidation resistance and thermal stability. They have created a series of polyurethanes with pendant carbonyl groups that exhibit improved UV resistance and weatherability[1]. BASF has also developed carbonyl-functionalized epoxy resins that show enhanced adhesion properties and chemical resistance[3]. These materials find applications in automotive coatings, construction materials, and industrial adhesives, where long-term durability is crucial.
Strengths: Extensive R&D capabilities, broad product portfolio, and global market presence. Weaknesses: High dependence on petrochemical feedstocks, potential environmental concerns with some chemical processes.
Core Innovations in Carbonyl-based Material Design
Carbonylphenacene compound, organic luminescent material, organic semiconductor material and method of producing carbonylphenacene compound
PatentInactiveJP2015178474A
Innovation
- Introduction of a carbonyl group (acyl group) into the phenacene structure to create carbonylphenacene compounds, which exhibit resistance to high voltage and oxygen, and possess excellent light-emitting properties.
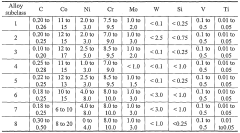
NANO-precipitation strengthened ultra-high strength corrosion resistant structural steels
PatentWO2004108970A2
Innovation
- Development of stainless steel alloys with a composition of 0.1-0.3% carbon, 8-17% cobalt, less than 10% nickel, 6-13% chromium, and nanoscale M2C carbides, which provide both ultrahigh strength and corrosion resistance without the need for cadmium or chromium coatings.
Environmental Impact of Carbonyl-based Materials
Carbonyl-based materials have become increasingly prevalent in various industries due to their unique properties and versatility. However, their widespread use has raised concerns about their environmental impact. The production, use, and disposal of carbonyl-based materials can have significant consequences for ecosystems and human health.
One of the primary environmental concerns associated with carbonyl-based materials is their potential for air pollution. During the manufacturing process, volatile organic compounds (VOCs) containing carbonyl groups can be released into the atmosphere. These compounds contribute to the formation of ground-level ozone and smog, which can have detrimental effects on air quality and respiratory health. Additionally, some carbonyl compounds, such as formaldehyde, are known carcinogens and can pose long-term health risks when released into the environment.
Water pollution is another critical issue related to carbonyl-based materials. Many of these compounds are water-soluble and can easily contaminate aquatic ecosystems. Industrial effluents containing carbonyl compounds can lead to the degradation of water quality, affecting aquatic life and potentially entering the food chain. Furthermore, the persistence of certain carbonyl-based materials in the environment can result in bioaccumulation, magnifying their impact on higher trophic levels.
The disposal of carbonyl-based materials presents additional environmental challenges. Many of these materials are not biodegradable and can persist in landfills for extended periods. As they break down, they may release harmful chemicals into the soil and groundwater. Incineration of carbonyl-containing waste can also lead to the formation of toxic byproducts, further contributing to air pollution.
However, it is important to note that not all environmental impacts of carbonyl-based materials are negative. Some carbonyl compounds play crucial roles in atmospheric chemistry and contribute to the natural carbon cycle. Additionally, certain carbonyl-based materials are being developed as environmentally friendly alternatives to more harmful substances, particularly in the field of green chemistry.
To mitigate the environmental impact of carbonyl-based materials, various strategies are being implemented. These include the development of more efficient production processes to reduce emissions, the use of catalytic converters and scrubbers to capture and neutralize harmful compounds, and the implementation of stricter regulations on the handling and disposal of these materials. Furthermore, research into biodegradable alternatives and the principles of green chemistry is ongoing, aiming to create more sustainable carbonyl-based materials with reduced environmental footprints.
One of the primary environmental concerns associated with carbonyl-based materials is their potential for air pollution. During the manufacturing process, volatile organic compounds (VOCs) containing carbonyl groups can be released into the atmosphere. These compounds contribute to the formation of ground-level ozone and smog, which can have detrimental effects on air quality and respiratory health. Additionally, some carbonyl compounds, such as formaldehyde, are known carcinogens and can pose long-term health risks when released into the environment.
Water pollution is another critical issue related to carbonyl-based materials. Many of these compounds are water-soluble and can easily contaminate aquatic ecosystems. Industrial effluents containing carbonyl compounds can lead to the degradation of water quality, affecting aquatic life and potentially entering the food chain. Furthermore, the persistence of certain carbonyl-based materials in the environment can result in bioaccumulation, magnifying their impact on higher trophic levels.
The disposal of carbonyl-based materials presents additional environmental challenges. Many of these materials are not biodegradable and can persist in landfills for extended periods. As they break down, they may release harmful chemicals into the soil and groundwater. Incineration of carbonyl-containing waste can also lead to the formation of toxic byproducts, further contributing to air pollution.
However, it is important to note that not all environmental impacts of carbonyl-based materials are negative. Some carbonyl compounds play crucial roles in atmospheric chemistry and contribute to the natural carbon cycle. Additionally, certain carbonyl-based materials are being developed as environmentally friendly alternatives to more harmful substances, particularly in the field of green chemistry.
To mitigate the environmental impact of carbonyl-based materials, various strategies are being implemented. These include the development of more efficient production processes to reduce emissions, the use of catalytic converters and scrubbers to capture and neutralize harmful compounds, and the implementation of stricter regulations on the handling and disposal of these materials. Furthermore, research into biodegradable alternatives and the principles of green chemistry is ongoing, aiming to create more sustainable carbonyl-based materials with reduced environmental footprints.
Scalability and Cost-effectiveness Analysis
The scalability and cost-effectiveness of carbonyl chemistry in enhancing material resilience are critical factors for its widespread adoption in industrial applications. The ability to scale up carbonyl-based processes while maintaining economic viability is essential for the commercial success of resilient materials.
Carbonyl chemistry offers several advantages in terms of scalability. The reactions involved are generally straightforward and can be performed under mild conditions, making them suitable for large-scale production. Many carbonyl compounds are readily available or can be synthesized from inexpensive precursors, facilitating the scaling of production processes. Additionally, the versatility of carbonyl chemistry allows for the development of modular synthesis routes, enabling efficient scale-up strategies.
However, challenges remain in optimizing reaction conditions and yields for industrial-scale production. Factors such as heat transfer, mixing efficiency, and reaction kinetics must be carefully considered when scaling up carbonyl-based processes. The development of continuous flow reactors and process intensification techniques can significantly improve the scalability of these reactions, allowing for higher throughput and better control over reaction parameters.
From a cost-effectiveness perspective, carbonyl chemistry presents both opportunities and challenges. The relatively low cost of many carbonyl precursors and the high atom economy of many carbonyl reactions contribute to the overall cost-effectiveness of this approach. Furthermore, the ability to fine-tune material properties through careful selection of carbonyl compounds and reaction conditions can lead to more efficient use of resources and reduced waste.
Nevertheless, the cost-effectiveness of carbonyl-based resilient materials must be evaluated in the context of their entire lifecycle. While initial production costs may be competitive, factors such as durability, maintenance requirements, and end-of-life disposal or recycling must be considered. In many cases, the enhanced resilience provided by carbonyl chemistry can lead to longer-lasting materials, potentially offsetting higher initial costs through reduced replacement and maintenance expenses.
To further improve the scalability and cost-effectiveness of carbonyl chemistry in material resilience applications, several strategies can be employed. These include the development of more efficient catalysts to enhance reaction rates and selectivity, the implementation of green chemistry principles to reduce environmental impact and associated costs, and the exploration of novel carbonyl precursors derived from renewable resources.
In conclusion, the scalability and cost-effectiveness of carbonyl chemistry in material resilience applications show significant promise. While challenges remain, ongoing research and technological advancements are likely to further improve the economic viability of this approach, paving the way for wider adoption in various industries seeking to enhance the durability and performance of their materials.
Carbonyl chemistry offers several advantages in terms of scalability. The reactions involved are generally straightforward and can be performed under mild conditions, making them suitable for large-scale production. Many carbonyl compounds are readily available or can be synthesized from inexpensive precursors, facilitating the scaling of production processes. Additionally, the versatility of carbonyl chemistry allows for the development of modular synthesis routes, enabling efficient scale-up strategies.
However, challenges remain in optimizing reaction conditions and yields for industrial-scale production. Factors such as heat transfer, mixing efficiency, and reaction kinetics must be carefully considered when scaling up carbonyl-based processes. The development of continuous flow reactors and process intensification techniques can significantly improve the scalability of these reactions, allowing for higher throughput and better control over reaction parameters.
From a cost-effectiveness perspective, carbonyl chemistry presents both opportunities and challenges. The relatively low cost of many carbonyl precursors and the high atom economy of many carbonyl reactions contribute to the overall cost-effectiveness of this approach. Furthermore, the ability to fine-tune material properties through careful selection of carbonyl compounds and reaction conditions can lead to more efficient use of resources and reduced waste.
Nevertheless, the cost-effectiveness of carbonyl-based resilient materials must be evaluated in the context of their entire lifecycle. While initial production costs may be competitive, factors such as durability, maintenance requirements, and end-of-life disposal or recycling must be considered. In many cases, the enhanced resilience provided by carbonyl chemistry can lead to longer-lasting materials, potentially offsetting higher initial costs through reduced replacement and maintenance expenses.
To further improve the scalability and cost-effectiveness of carbonyl chemistry in material resilience applications, several strategies can be employed. These include the development of more efficient catalysts to enhance reaction rates and selectivity, the implementation of green chemistry principles to reduce environmental impact and associated costs, and the exploration of novel carbonyl precursors derived from renewable resources.
In conclusion, the scalability and cost-effectiveness of carbonyl chemistry in material resilience applications show significant promise. While challenges remain, ongoing research and technological advancements are likely to further improve the economic viability of this approach, paving the way for wider adoption in various industries seeking to enhance the durability and performance of their materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!