Carbonyl Group Contributions to Safer Product Manufacturing
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Safety Goals
The development of safer product manufacturing processes involving carbonyl groups is a critical objective in the chemical industry. This goal encompasses several key aspects aimed at mitigating risks associated with carbonyl compounds while maintaining or enhancing production efficiency. One primary focus is the reduction of exposure to potentially harmful carbonyl-containing substances during manufacturing operations. This involves implementing advanced containment systems, improving ventilation, and utilizing closed-loop processes to minimize worker contact with volatile carbonyl compounds.
Another crucial safety goal is the optimization of reaction conditions to prevent the formation of hazardous by-products. This includes fine-tuning temperature, pressure, and catalyst parameters to ensure selective carbonyl reactions while suppressing unwanted side reactions that could lead to the generation of toxic or unstable intermediates. Additionally, there is a strong emphasis on developing alternative synthetic routes that utilize less hazardous carbonyl precursors or avoid the use of carbonyl groups altogether when possible.
The integration of real-time monitoring systems for carbonyl-related processes is another key objective. These systems aim to detect and alert operators to any deviations in reaction conditions or the presence of unexpected carbonyl species, allowing for immediate corrective actions. This approach not only enhances safety but also contributes to overall process control and product quality.
Waste management and environmental protection are also integral to carbonyl safety goals. Efforts are directed towards developing more efficient purification and separation techniques to minimize the release of carbonyl-containing waste streams. This includes the implementation of advanced oxidation processes and catalytic degradation methods to treat carbonyl-rich effluents before discharge.
Furthermore, there is a growing focus on the design of inherently safer carbonyl chemistry. This involves the application of green chemistry principles to create processes that are less prone to runaway reactions, reduce the potential for accidental releases, and minimize the overall hazard profile of carbonyl-based operations. The development of novel protective groups and reaction methodologies that allow for milder conditions and increased selectivity is a key part of this strategy.
Lastly, the carbonyl safety goals extend to the realm of product design, where efforts are made to reduce the presence of residual carbonyl compounds in final products. This is particularly important in industries such as pharmaceuticals, food additives, and consumer goods, where trace amounts of carbonyl impurities can have significant health implications. Advanced analytical techniques and stringent quality control measures are being developed to ensure the purity and safety of carbonyl-derived products.
Another crucial safety goal is the optimization of reaction conditions to prevent the formation of hazardous by-products. This includes fine-tuning temperature, pressure, and catalyst parameters to ensure selective carbonyl reactions while suppressing unwanted side reactions that could lead to the generation of toxic or unstable intermediates. Additionally, there is a strong emphasis on developing alternative synthetic routes that utilize less hazardous carbonyl precursors or avoid the use of carbonyl groups altogether when possible.
The integration of real-time monitoring systems for carbonyl-related processes is another key objective. These systems aim to detect and alert operators to any deviations in reaction conditions or the presence of unexpected carbonyl species, allowing for immediate corrective actions. This approach not only enhances safety but also contributes to overall process control and product quality.
Waste management and environmental protection are also integral to carbonyl safety goals. Efforts are directed towards developing more efficient purification and separation techniques to minimize the release of carbonyl-containing waste streams. This includes the implementation of advanced oxidation processes and catalytic degradation methods to treat carbonyl-rich effluents before discharge.
Furthermore, there is a growing focus on the design of inherently safer carbonyl chemistry. This involves the application of green chemistry principles to create processes that are less prone to runaway reactions, reduce the potential for accidental releases, and minimize the overall hazard profile of carbonyl-based operations. The development of novel protective groups and reaction methodologies that allow for milder conditions and increased selectivity is a key part of this strategy.
Lastly, the carbonyl safety goals extend to the realm of product design, where efforts are made to reduce the presence of residual carbonyl compounds in final products. This is particularly important in industries such as pharmaceuticals, food additives, and consumer goods, where trace amounts of carbonyl impurities can have significant health implications. Advanced analytical techniques and stringent quality control measures are being developed to ensure the purity and safety of carbonyl-derived products.
Market Demand Analysis
The market demand for safer product manufacturing processes involving carbonyl group contributions has been steadily increasing in recent years. This growth is driven by several factors, including stricter regulations on chemical safety, growing consumer awareness of product safety, and the need for more sustainable manufacturing practices.
In the pharmaceutical industry, there is a significant demand for safer synthesis methods involving carbonyl groups. These functional groups are crucial in the production of many active pharmaceutical ingredients (APIs). The global pharmaceutical market, valued at over $1.4 trillion in 2021, is expected to continue growing, creating a substantial demand for improved manufacturing processes that enhance safety and reduce environmental impact.
The personal care and cosmetics industry also presents a considerable market for safer carbonyl-based product manufacturing. With consumers becoming more conscious of ingredient safety, there is a growing preference for products made using safer, more environmentally friendly processes. The global cosmetics market, which exceeded $380 billion in 2021, is projected to expand further, driving the need for innovative and safer manufacturing techniques.
In the food and beverage sector, carbonyl compounds play a vital role in flavoring and aroma production. As consumers increasingly demand clean label products, there is a rising need for safer and more natural methods of incorporating these compounds. The global food flavors market, valued at around $16 billion in 2021, is expected to grow significantly, creating opportunities for safer manufacturing processes.
The polymer and plastics industry, another major sector utilizing carbonyl chemistry, is experiencing a shift towards safer and more sustainable production methods. With the global plastics market projected to reach $750 billion by 2028, there is a substantial demand for improved manufacturing processes that reduce health and environmental risks associated with carbonyl compounds.
Environmental concerns and regulatory pressures are also driving market demand across industries. Governments worldwide are implementing stricter regulations on chemical safety and emissions, pushing companies to invest in safer manufacturing processes. This regulatory landscape is creating a robust market for technologies and solutions that can improve the safety profile of carbonyl-based product manufacturing.
The market demand is further bolstered by the growing emphasis on occupational safety in manufacturing facilities. Companies are increasingly recognizing the long-term benefits of investing in safer processes, including reduced liability risks, improved worker health, and enhanced corporate reputation.
In the pharmaceutical industry, there is a significant demand for safer synthesis methods involving carbonyl groups. These functional groups are crucial in the production of many active pharmaceutical ingredients (APIs). The global pharmaceutical market, valued at over $1.4 trillion in 2021, is expected to continue growing, creating a substantial demand for improved manufacturing processes that enhance safety and reduce environmental impact.
The personal care and cosmetics industry also presents a considerable market for safer carbonyl-based product manufacturing. With consumers becoming more conscious of ingredient safety, there is a growing preference for products made using safer, more environmentally friendly processes. The global cosmetics market, which exceeded $380 billion in 2021, is projected to expand further, driving the need for innovative and safer manufacturing techniques.
In the food and beverage sector, carbonyl compounds play a vital role in flavoring and aroma production. As consumers increasingly demand clean label products, there is a rising need for safer and more natural methods of incorporating these compounds. The global food flavors market, valued at around $16 billion in 2021, is expected to grow significantly, creating opportunities for safer manufacturing processes.
The polymer and plastics industry, another major sector utilizing carbonyl chemistry, is experiencing a shift towards safer and more sustainable production methods. With the global plastics market projected to reach $750 billion by 2028, there is a substantial demand for improved manufacturing processes that reduce health and environmental risks associated with carbonyl compounds.
Environmental concerns and regulatory pressures are also driving market demand across industries. Governments worldwide are implementing stricter regulations on chemical safety and emissions, pushing companies to invest in safer manufacturing processes. This regulatory landscape is creating a robust market for technologies and solutions that can improve the safety profile of carbonyl-based product manufacturing.
The market demand is further bolstered by the growing emphasis on occupational safety in manufacturing facilities. Companies are increasingly recognizing the long-term benefits of investing in safer processes, including reduced liability risks, improved worker health, and enhanced corporate reputation.
Carbonyl Challenges
The carbonyl group, a fundamental functional group in organic chemistry, presents significant challenges in product manufacturing, particularly concerning safety aspects. One of the primary issues is the reactivity of carbonyl compounds, which can lead to unwanted side reactions during production processes. This reactivity stems from the polarized nature of the carbon-oxygen double bond, making carbonyl groups susceptible to nucleophilic attacks and various other chemical transformations.
In the context of safer product manufacturing, the volatility of many carbonyl-containing compounds poses a considerable challenge. Aldehydes and ketones, common carbonyl-bearing molecules, often have low boiling points and high vapor pressures. This characteristic increases the risk of worker exposure through inhalation, potentially leading to respiratory irritation or more severe health effects. Moreover, the flammability of many carbonyl compounds adds another layer of safety concerns in manufacturing environments.
The tendency of carbonyl groups to undergo oxidation reactions is another significant challenge. This propensity can lead to product degradation during storage or use, affecting the shelf life and quality of manufactured goods. In some cases, oxidation of carbonyl compounds can result in the formation of peroxides, which are highly unstable and potentially explosive, posing serious safety risks in manufacturing facilities.
Carbonyl compounds are also known for their ability to form Schiff bases and undergo aldol condensations. While these reactions are valuable in certain synthetic processes, they can be problematic in product manufacturing. Uncontrolled Schiff base formation or aldol condensation can lead to unwanted polymerization, affecting product purity and potentially causing equipment fouling or blockages in production lines.
The environmental impact of carbonyl-containing compounds is an additional challenge in safer product manufacturing. Many of these compounds are classified as volatile organic compounds (VOCs), contributing to air pollution and potentially harming ecosystems. Stricter environmental regulations are pushing manufacturers to find alternatives or develop more efficient containment and treatment methods for carbonyl emissions.
Lastly, the potential for carbonyl compounds to act as sensitizers or irritants presents challenges in ensuring worker safety and product safety for end-users. Some carbonyl-containing substances can cause allergic reactions or skin irritation upon repeated exposure, necessitating careful handling procedures and appropriate personal protective equipment in manufacturing settings.
In the context of safer product manufacturing, the volatility of many carbonyl-containing compounds poses a considerable challenge. Aldehydes and ketones, common carbonyl-bearing molecules, often have low boiling points and high vapor pressures. This characteristic increases the risk of worker exposure through inhalation, potentially leading to respiratory irritation or more severe health effects. Moreover, the flammability of many carbonyl compounds adds another layer of safety concerns in manufacturing environments.
The tendency of carbonyl groups to undergo oxidation reactions is another significant challenge. This propensity can lead to product degradation during storage or use, affecting the shelf life and quality of manufactured goods. In some cases, oxidation of carbonyl compounds can result in the formation of peroxides, which are highly unstable and potentially explosive, posing serious safety risks in manufacturing facilities.
Carbonyl compounds are also known for their ability to form Schiff bases and undergo aldol condensations. While these reactions are valuable in certain synthetic processes, they can be problematic in product manufacturing. Uncontrolled Schiff base formation or aldol condensation can lead to unwanted polymerization, affecting product purity and potentially causing equipment fouling or blockages in production lines.
The environmental impact of carbonyl-containing compounds is an additional challenge in safer product manufacturing. Many of these compounds are classified as volatile organic compounds (VOCs), contributing to air pollution and potentially harming ecosystems. Stricter environmental regulations are pushing manufacturers to find alternatives or develop more efficient containment and treatment methods for carbonyl emissions.
Lastly, the potential for carbonyl compounds to act as sensitizers or irritants presents challenges in ensuring worker safety and product safety for end-users. Some carbonyl-containing substances can cause allergic reactions or skin irritation upon repeated exposure, necessitating careful handling procedures and appropriate personal protective equipment in manufacturing settings.
Current Safety Solutions
01 Safety measures for handling carbonyl compounds
Implementing proper safety protocols when handling carbonyl compounds is crucial. This includes using appropriate personal protective equipment, ensuring adequate ventilation, and following safe storage and disposal procedures. These measures help minimize exposure risks and potential health hazards associated with carbonyl groups.- Safety measures for handling carbonyl compounds: Implementing proper safety protocols when working with carbonyl compounds is crucial. This includes using appropriate personal protective equipment, ensuring adequate ventilation, and following safe handling procedures to minimize exposure risks and potential health hazards associated with these compounds.
- Reducing toxicity of carbonyl groups: Various methods can be employed to reduce the toxicity of carbonyl groups in chemical compounds. These may include chemical modifications, encapsulation techniques, or the use of less toxic alternatives to achieve similar functional properties while improving overall safety profiles.
- Environmental impact of carbonyl compounds: Assessing and mitigating the environmental impact of carbonyl compounds is essential for ensuring ecological safety. This involves developing environmentally friendly production processes, implementing proper disposal methods, and conducting thorough environmental risk assessments.
- Analytical methods for carbonyl group safety assessment: Advanced analytical techniques are employed to assess the safety of carbonyl groups in various applications. These methods may include spectroscopic analysis, chromatography, and other sophisticated tools to accurately determine the presence, concentration, and potential risks associated with carbonyl compounds.
- Regulatory compliance for carbonyl group safety: Adhering to regulatory guidelines and standards is crucial for ensuring the safe use of carbonyl compounds in various industries. This includes compliance with occupational safety regulations, product safety standards, and environmental protection laws to minimize risks associated with carbonyl groups.
02 Reducing carbonyl group reactivity
Methods to reduce the reactivity of carbonyl groups can enhance safety. This may involve chemical modifications, such as forming derivatives or using protective groups, to decrease the compound's reactivity and potential toxicity. These techniques can improve the handling and storage safety of carbonyl-containing substances.Expand Specific Solutions03 Environmental impact assessment of carbonyl compounds
Evaluating the environmental impact of carbonyl compounds is essential for ensuring their safe use and disposal. This includes studying their biodegradability, potential for bioaccumulation, and effects on aquatic and terrestrial ecosystems. Such assessments help in developing environmentally friendly practices and regulations.Expand Specific Solutions04 Toxicological studies on carbonyl group-containing substances
Conducting comprehensive toxicological studies on substances containing carbonyl groups is crucial for understanding their potential health effects. These studies may include acute and chronic toxicity tests, carcinogenicity assessments, and investigations into potential mutagenic or teratogenic effects. The results inform safety guidelines and regulations.Expand Specific Solutions05 Development of safer alternatives to hazardous carbonyl compounds
Research into developing safer alternatives to hazardous carbonyl compounds is ongoing. This involves designing molecules with similar functional properties but reduced toxicity or environmental impact. Such efforts contribute to the overall safety of industrial processes and consumer products that traditionally use carbonyl-containing substances.Expand Specific Solutions
Key Industry Players
The research on carbonyl group contributions to safer product manufacturing is in a developing stage, with growing market potential as industries focus on sustainable and safer chemical processes. The technology's maturity varies across different sectors, with some companies leading the way. Key players like Takeda Pharmaceutical, Sumitomo Chemical, and UBE Corp. are investing in research and development to advance this field. The market is characterized by a mix of established chemical companies and innovative startups, such as Cascat GmbH, working on novel approaches. As environmental and safety regulations tighten globally, the demand for safer manufacturing processes is expected to drive further growth and innovation in this area.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda Pharmaceutical has made significant advancements in the safe handling and manipulation of carbonyl-containing compounds in drug manufacturing. They have developed a suite of novel protecting group strategies specifically designed for sensitive carbonyl functionalities in complex pharmaceutical intermediates [8]. Takeda has also implemented continuous flow chemistry techniques for carbonyl transformations, allowing for improved process safety and reduced exposure risks [10]. The company has invested in the development of green oxidation methods for the synthesis of carbonyl compounds, utilizing environmentally benign oxidants and catalysts [12]. Additionally, Takeda has pioneered the use of photochemistry for carbonyl manipulations, offering milder reaction conditions and improved selectivity [14].
Strengths: Strong expertise in pharmaceutical chemistry, focus on process safety, and a robust drug development pipeline. Weaknesses: High regulatory hurdles for novel manufacturing processes and potential scalability challenges.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has made significant strides in research on carbonyl group contributions to safer product manufacturing. The company has developed a proprietary process for the synthesis of carbonyl-containing compounds using supercritical carbon dioxide as a solvent, which reduces the use of toxic organic solvents and improves overall safety [2]. They have also implemented advanced process analytical technology (PAT) for real-time monitoring of carbonyl reactions, enhancing process control and safety [4]. Furthermore, Sumitomo has invested in the development of novel protective group strategies for carbonyl functionalities, allowing for safer handling and manipulation of sensitive intermediates in complex synthesis routes [6].
Strengths: Strong focus on green chemistry, advanced process technologies, and a robust patent portfolio. Weaknesses: Potential scalability issues for some novel technologies and high initial investment costs.
Carbonyl Innovations
The preparation of organic compounds containing a carbonyl group and compositions for use therein
PatentInactiveGB1025679A
Innovation
- A method involving a contact agent comprising molybdenum trioxide or heteropolyacids and Pt or Pd, with specific weight percentages, is used to convert hydrocarbons, either in the gas phase or solution, with optional support and oxidizing agents to produce carbonyl compounds efficiently.
Remediation of physiologically active compounds from waste water
PatentInactiveUS20200140290A1
Innovation
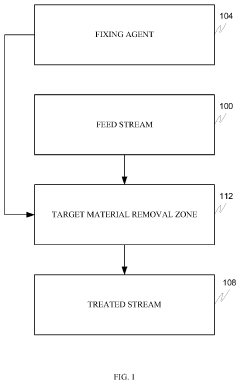
- A process involving rare earth metals as soluble or insoluble fixing agents to form insoluble target material-containing agents, which precipitate out and can be easily removed from the water stream, thereby stabilizing and eliminating these materials.
Regulatory Framework
The regulatory framework surrounding carbonyl group contributions in product manufacturing is complex and multifaceted, involving various governmental agencies and international bodies. At the forefront of this framework is the Occupational Safety and Health Administration (OSHA), which sets and enforces standards to ensure safe and healthful working conditions. OSHA has established Permissible Exposure Limits (PELs) for many carbonyl compounds, such as formaldehyde and acetone, to protect workers from harmful exposure levels.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating carbonyl compounds under the Toxic Substances Control Act (TSCA). The TSCA empowers the EPA to require reporting, record-keeping, and testing of chemicals that may pose environmental or health risks. Additionally, the EPA's Clean Air Act regulates emissions of volatile organic compounds (VOCs), many of which contain carbonyl groups, from industrial facilities.
On an international level, the European Union's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation impacts manufacturers worldwide. REACH requires companies to register chemical substances manufactured or imported into the EU in quantities of one tonne or more per year, including many carbonyl-containing compounds. This regulation aims to improve the protection of human health and the environment through better and earlier identification of the intrinsic properties of chemical substances.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication. Many carbonyl compounds are classified under GHS based on their health and environmental hazards, influencing their labeling and safety data sheet requirements globally.
In the realm of consumer product safety, the Consumer Product Safety Commission (CPSC) in the United States regulates the use of certain carbonyl compounds in consumer goods. For instance, the CPSC has set limits on formaldehyde emissions from composite wood products to protect consumers from potential health risks.
The Food and Drug Administration (FDA) oversees the regulation of carbonyl compounds in food additives, pharmaceuticals, and cosmetics. The FDA's Good Manufacturing Practices (GMP) guidelines include specific requirements for the handling and use of carbonyl-containing substances in these industries.
As research on carbonyl group contributions to safer product manufacturing progresses, regulatory bodies are likely to update their frameworks. This may include revising exposure limits, implementing new testing requirements, or introducing regulations for emerging carbonyl compounds of concern. Manufacturers must stay informed about these evolving regulations to ensure compliance and maintain product safety.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating carbonyl compounds under the Toxic Substances Control Act (TSCA). The TSCA empowers the EPA to require reporting, record-keeping, and testing of chemicals that may pose environmental or health risks. Additionally, the EPA's Clean Air Act regulates emissions of volatile organic compounds (VOCs), many of which contain carbonyl groups, from industrial facilities.
On an international level, the European Union's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation impacts manufacturers worldwide. REACH requires companies to register chemical substances manufactured or imported into the EU in quantities of one tonne or more per year, including many carbonyl-containing compounds. This regulation aims to improve the protection of human health and the environment through better and earlier identification of the intrinsic properties of chemical substances.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication. Many carbonyl compounds are classified under GHS based on their health and environmental hazards, influencing their labeling and safety data sheet requirements globally.
In the realm of consumer product safety, the Consumer Product Safety Commission (CPSC) in the United States regulates the use of certain carbonyl compounds in consumer goods. For instance, the CPSC has set limits on formaldehyde emissions from composite wood products to protect consumers from potential health risks.
The Food and Drug Administration (FDA) oversees the regulation of carbonyl compounds in food additives, pharmaceuticals, and cosmetics. The FDA's Good Manufacturing Practices (GMP) guidelines include specific requirements for the handling and use of carbonyl-containing substances in these industries.
As research on carbonyl group contributions to safer product manufacturing progresses, regulatory bodies are likely to update their frameworks. This may include revising exposure limits, implementing new testing requirements, or introducing regulations for emerging carbonyl compounds of concern. Manufacturers must stay informed about these evolving regulations to ensure compliance and maintain product safety.
Environmental Impact
The environmental impact of carbonyl group contributions in product manufacturing is a critical consideration for safer and more sustainable industrial practices. Carbonyl compounds, characterized by the presence of a carbon-oxygen double bond, are ubiquitous in various industrial processes and products. Their reactivity and versatility make them essential in many chemical reactions, but also pose potential risks to the environment.
One of the primary environmental concerns associated with carbonyl groups is their potential to form volatile organic compounds (VOCs). When released into the atmosphere, these compounds can contribute to the formation of ground-level ozone and smog, leading to air quality degradation and associated health risks. Additionally, some carbonyl compounds, such as formaldehyde and acetaldehyde, are known air toxics that can have direct adverse effects on human health and ecosystems.
Water pollution is another significant environmental impact of carbonyl-containing compounds. Many of these substances are water-soluble and can easily enter aquatic ecosystems through industrial effluents or accidental spills. In water bodies, carbonyl compounds can undergo various reactions, potentially forming harmful byproducts or disrupting aquatic life cycles. Some carbonyl derivatives, like certain aldehydes, have been shown to be toxic to aquatic organisms even at low concentrations.
The persistence and bioaccumulation potential of certain carbonyl compounds in the environment are also of concern. While many simple carbonyl compounds are biodegradable, more complex or halogenated derivatives may persist in the environment for extended periods. This persistence can lead to long-term ecological impacts and potential bioaccumulation in food chains, affecting wildlife and potentially human health through indirect exposure.
In the context of safer product manufacturing, addressing the environmental impact of carbonyl groups requires a multifaceted approach. This includes developing greener synthesis routes that minimize the use or generation of harmful carbonyl compounds, implementing more efficient containment and treatment systems for industrial emissions and effluents, and exploring alternative compounds or technologies that can replace high-impact carbonyl-based processes.
Research into the environmental fate and transport of carbonyl compounds is crucial for understanding their long-term impacts and developing effective mitigation strategies. This includes studying their atmospheric chemistry, aquatic transformations, and interactions with various environmental matrices. Such research can inform the development of more accurate risk assessment models and guide regulatory decisions aimed at protecting environmental and human health.
One of the primary environmental concerns associated with carbonyl groups is their potential to form volatile organic compounds (VOCs). When released into the atmosphere, these compounds can contribute to the formation of ground-level ozone and smog, leading to air quality degradation and associated health risks. Additionally, some carbonyl compounds, such as formaldehyde and acetaldehyde, are known air toxics that can have direct adverse effects on human health and ecosystems.
Water pollution is another significant environmental impact of carbonyl-containing compounds. Many of these substances are water-soluble and can easily enter aquatic ecosystems through industrial effluents or accidental spills. In water bodies, carbonyl compounds can undergo various reactions, potentially forming harmful byproducts or disrupting aquatic life cycles. Some carbonyl derivatives, like certain aldehydes, have been shown to be toxic to aquatic organisms even at low concentrations.
The persistence and bioaccumulation potential of certain carbonyl compounds in the environment are also of concern. While many simple carbonyl compounds are biodegradable, more complex or halogenated derivatives may persist in the environment for extended periods. This persistence can lead to long-term ecological impacts and potential bioaccumulation in food chains, affecting wildlife and potentially human health through indirect exposure.
In the context of safer product manufacturing, addressing the environmental impact of carbonyl groups requires a multifaceted approach. This includes developing greener synthesis routes that minimize the use or generation of harmful carbonyl compounds, implementing more efficient containment and treatment systems for industrial emissions and effluents, and exploring alternative compounds or technologies that can replace high-impact carbonyl-based processes.
Research into the environmental fate and transport of carbonyl compounds is crucial for understanding their long-term impacts and developing effective mitigation strategies. This includes studying their atmospheric chemistry, aquatic transformations, and interactions with various environmental matrices. Such research can inform the development of more accurate risk assessment models and guide regulatory decisions aimed at protecting environmental and human health.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!