Carbonyl Processes for Cost-Effective Manufacturing Approaches
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Process Evolution and Objectives
Carbonyl processes have played a pivotal role in the manufacturing industry since their inception in the early 20th century. These processes, which involve the reaction of metal carbonyls with various substrates, have evolved significantly over the years, driven by the need for more efficient and cost-effective manufacturing approaches.
The evolution of carbonyl processes can be traced back to the discovery of nickel tetracarbonyl by Ludwig Mond in 1890. This breakthrough laid the foundation for the development of various carbonyl-based manufacturing techniques. In the following decades, researchers and industry professionals worked tirelessly to expand the scope and applicability of these processes, leading to the development of new metal carbonyls and innovative reaction pathways.
One of the key milestones in the evolution of carbonyl processes was the commercialization of the Mond process for nickel purification in the early 1900s. This process demonstrated the potential of carbonyl chemistry in industrial applications and paved the way for further advancements. Subsequently, the development of iron pentacarbonyl and its use in the manufacture of high-purity iron powder marked another significant step forward.
As the understanding of carbonyl chemistry deepened, researchers began exploring its potential in the synthesis of various organic compounds. This led to the development of hydroformylation processes, also known as oxo synthesis, which became a cornerstone of industrial organic chemistry. The ability to convert olefins into aldehydes opened up new avenues for the production of valuable chemicals and intermediates.
In recent years, the focus of carbonyl process research has shifted towards improving efficiency, reducing environmental impact, and enhancing cost-effectiveness. This has led to the exploration of novel catalysts, the optimization of reaction conditions, and the development of more sustainable manufacturing approaches. The integration of advanced technologies, such as continuous flow reactors and process intensification techniques, has further contributed to the evolution of carbonyl processes.
The primary objectives of current research in carbonyl processes for cost-effective manufacturing approaches are multifaceted. Firstly, there is a strong emphasis on developing more selective and efficient catalysts that can operate under milder conditions, thereby reducing energy consumption and improving overall process economics. Secondly, researchers are exploring ways to expand the substrate scope of carbonyl processes, enabling the synthesis of a wider range of valuable products.
Another key objective is the development of greener and more sustainable carbonyl processes. This includes the use of renewable feedstocks, the minimization of waste generation, and the implementation of recycling strategies for metal carbonyls. Additionally, there is a growing interest in the application of carbonyl chemistry in the field of nanotechnology, particularly for the synthesis of metal nanoparticles with controlled size and morphology.
The evolution of carbonyl processes can be traced back to the discovery of nickel tetracarbonyl by Ludwig Mond in 1890. This breakthrough laid the foundation for the development of various carbonyl-based manufacturing techniques. In the following decades, researchers and industry professionals worked tirelessly to expand the scope and applicability of these processes, leading to the development of new metal carbonyls and innovative reaction pathways.
One of the key milestones in the evolution of carbonyl processes was the commercialization of the Mond process for nickel purification in the early 1900s. This process demonstrated the potential of carbonyl chemistry in industrial applications and paved the way for further advancements. Subsequently, the development of iron pentacarbonyl and its use in the manufacture of high-purity iron powder marked another significant step forward.
As the understanding of carbonyl chemistry deepened, researchers began exploring its potential in the synthesis of various organic compounds. This led to the development of hydroformylation processes, also known as oxo synthesis, which became a cornerstone of industrial organic chemistry. The ability to convert olefins into aldehydes opened up new avenues for the production of valuable chemicals and intermediates.
In recent years, the focus of carbonyl process research has shifted towards improving efficiency, reducing environmental impact, and enhancing cost-effectiveness. This has led to the exploration of novel catalysts, the optimization of reaction conditions, and the development of more sustainable manufacturing approaches. The integration of advanced technologies, such as continuous flow reactors and process intensification techniques, has further contributed to the evolution of carbonyl processes.
The primary objectives of current research in carbonyl processes for cost-effective manufacturing approaches are multifaceted. Firstly, there is a strong emphasis on developing more selective and efficient catalysts that can operate under milder conditions, thereby reducing energy consumption and improving overall process economics. Secondly, researchers are exploring ways to expand the substrate scope of carbonyl processes, enabling the synthesis of a wider range of valuable products.
Another key objective is the development of greener and more sustainable carbonyl processes. This includes the use of renewable feedstocks, the minimization of waste generation, and the implementation of recycling strategies for metal carbonyls. Additionally, there is a growing interest in the application of carbonyl chemistry in the field of nanotechnology, particularly for the synthesis of metal nanoparticles with controlled size and morphology.
Market Analysis for Carbonyl-Based Products
The carbonyl process, a key manufacturing approach in the chemical industry, has garnered significant attention due to its potential for cost-effective production of various valuable compounds. Market analysis reveals a growing demand for carbonyl-based products across multiple sectors, including pharmaceuticals, agrochemicals, and specialty chemicals. The global market for carbonyl compounds is projected to expand steadily, driven by increasing applications in diverse industries and the push for more sustainable manufacturing processes.
In the pharmaceutical sector, carbonyl-based intermediates play a crucial role in the synthesis of active pharmaceutical ingredients (APIs). The rising prevalence of chronic diseases and the continuous development of new drugs are fueling the demand for these compounds. Additionally, the agrochemical industry relies heavily on carbonyl chemistry for the production of pesticides and herbicides, contributing to market growth as global agricultural needs increase.
The specialty chemicals segment, encompassing flavors, fragrances, and advanced materials, represents another significant market for carbonyl-based products. Consumer preferences for natural and organic products have led to increased research and development in bio-based carbonyl compounds, opening new avenues for market expansion.
Geographically, Asia-Pacific dominates the carbonyl compounds market, with China and India emerging as major production hubs due to their robust chemical manufacturing infrastructure and lower production costs. North America and Europe follow, driven by strong demand from the pharmaceutical and specialty chemicals sectors.
Market trends indicate a shift towards greener and more sustainable carbonyl processes. This includes the development of catalytic systems that enable more efficient and environmentally friendly reactions, as well as the exploration of bio-based feedstocks for carbonyl compound production. These innovations are expected to create new opportunities in the market and potentially disrupt traditional manufacturing approaches.
The competitive landscape of the carbonyl-based products market is characterized by a mix of large multinational corporations and specialized chemical companies. Key players are investing heavily in research and development to improve process efficiency and develop novel applications for carbonyl compounds. Strategic partnerships and collaborations between industry and academia are becoming increasingly common, fostering innovation and accelerating the commercialization of new technologies.
Despite the positive outlook, the market faces challenges such as volatile raw material prices and stringent environmental regulations. However, these challenges also drive innovation in process technologies and catalysis, potentially leading to more cost-effective and sustainable manufacturing approaches in the long term.
In the pharmaceutical sector, carbonyl-based intermediates play a crucial role in the synthesis of active pharmaceutical ingredients (APIs). The rising prevalence of chronic diseases and the continuous development of new drugs are fueling the demand for these compounds. Additionally, the agrochemical industry relies heavily on carbonyl chemistry for the production of pesticides and herbicides, contributing to market growth as global agricultural needs increase.
The specialty chemicals segment, encompassing flavors, fragrances, and advanced materials, represents another significant market for carbonyl-based products. Consumer preferences for natural and organic products have led to increased research and development in bio-based carbonyl compounds, opening new avenues for market expansion.
Geographically, Asia-Pacific dominates the carbonyl compounds market, with China and India emerging as major production hubs due to their robust chemical manufacturing infrastructure and lower production costs. North America and Europe follow, driven by strong demand from the pharmaceutical and specialty chemicals sectors.
Market trends indicate a shift towards greener and more sustainable carbonyl processes. This includes the development of catalytic systems that enable more efficient and environmentally friendly reactions, as well as the exploration of bio-based feedstocks for carbonyl compound production. These innovations are expected to create new opportunities in the market and potentially disrupt traditional manufacturing approaches.
The competitive landscape of the carbonyl-based products market is characterized by a mix of large multinational corporations and specialized chemical companies. Key players are investing heavily in research and development to improve process efficiency and develop novel applications for carbonyl compounds. Strategic partnerships and collaborations between industry and academia are becoming increasingly common, fostering innovation and accelerating the commercialization of new technologies.
Despite the positive outlook, the market faces challenges such as volatile raw material prices and stringent environmental regulations. However, these challenges also drive innovation in process technologies and catalysis, potentially leading to more cost-effective and sustainable manufacturing approaches in the long term.
Current Challenges in Carbonyl Manufacturing
Carbonyl manufacturing processes face several significant challenges that hinder their cost-effectiveness and widespread adoption in industrial applications. One of the primary obstacles is the high energy consumption associated with traditional carbonyl synthesis methods. These processes often require elevated temperatures and pressures, resulting in substantial operational costs and reduced energy efficiency.
Another major challenge lies in the selectivity and yield of carbonyl compounds. Many current manufacturing approaches struggle to achieve high selectivity, leading to the formation of unwanted by-products. This not only reduces the overall yield of the desired carbonyl compounds but also necessitates additional purification steps, further increasing production costs and complexity.
The use of expensive catalysts presents another hurdle in carbonyl manufacturing. Noble metal catalysts, such as palladium and platinum, are commonly employed to facilitate carbonyl formation reactions. However, their high cost and limited availability pose significant economic challenges, especially for large-scale production.
Environmental concerns also play a crucial role in the current challenges faced by carbonyl manufacturing. Traditional processes often involve the use of toxic reagents and generate hazardous waste streams, raising environmental and safety issues. Stricter regulations and growing environmental awareness have put pressure on manufacturers to develop greener and more sustainable production methods.
Scalability remains a persistent challenge in carbonyl manufacturing. While many processes work efficiently at laboratory scales, translating them to industrial-scale production often encounters difficulties. Issues such as heat and mass transfer limitations, reactor design constraints, and process control complexities can significantly impact the feasibility and economics of large-scale carbonyl synthesis.
The volatility of raw material prices, particularly for petroleum-based feedstocks, adds another layer of complexity to carbonyl manufacturing. Fluctuations in the cost of starting materials can have a substantial impact on the overall production costs, making it challenging for manufacturers to maintain consistent pricing and profitability.
Lastly, the development of novel, cost-effective carbonyl manufacturing approaches is hindered by the limited understanding of reaction mechanisms and kinetics in complex industrial settings. This knowledge gap makes it difficult to optimize processes and design more efficient catalysts, further impeding progress towards more economical production methods.
Another major challenge lies in the selectivity and yield of carbonyl compounds. Many current manufacturing approaches struggle to achieve high selectivity, leading to the formation of unwanted by-products. This not only reduces the overall yield of the desired carbonyl compounds but also necessitates additional purification steps, further increasing production costs and complexity.
The use of expensive catalysts presents another hurdle in carbonyl manufacturing. Noble metal catalysts, such as palladium and platinum, are commonly employed to facilitate carbonyl formation reactions. However, their high cost and limited availability pose significant economic challenges, especially for large-scale production.
Environmental concerns also play a crucial role in the current challenges faced by carbonyl manufacturing. Traditional processes often involve the use of toxic reagents and generate hazardous waste streams, raising environmental and safety issues. Stricter regulations and growing environmental awareness have put pressure on manufacturers to develop greener and more sustainable production methods.
Scalability remains a persistent challenge in carbonyl manufacturing. While many processes work efficiently at laboratory scales, translating them to industrial-scale production often encounters difficulties. Issues such as heat and mass transfer limitations, reactor design constraints, and process control complexities can significantly impact the feasibility and economics of large-scale carbonyl synthesis.
The volatility of raw material prices, particularly for petroleum-based feedstocks, adds another layer of complexity to carbonyl manufacturing. Fluctuations in the cost of starting materials can have a substantial impact on the overall production costs, making it challenging for manufacturers to maintain consistent pricing and profitability.
Lastly, the development of novel, cost-effective carbonyl manufacturing approaches is hindered by the limited understanding of reaction mechanisms and kinetics in complex industrial settings. This knowledge gap makes it difficult to optimize processes and design more efficient catalysts, further impeding progress towards more economical production methods.
Cost-Effective Carbonyl Process Solutions
01 Process optimization for cost-effectiveness
Optimizing carbonyl processes involves improving reaction conditions, catalysts, and process parameters to enhance yield and reduce waste. This can include developing more efficient catalysts, optimizing reaction temperatures and pressures, and implementing continuous flow processes. Such optimizations can significantly reduce production costs and improve overall process economics.- Process optimization for cost-effective carbonyl production: Optimizing carbonyl processes involves improving reaction conditions, catalysts, and process parameters to enhance yield and reduce operational costs. This includes developing more efficient catalysts, optimizing reaction temperatures and pressures, and implementing advanced process control systems to minimize waste and energy consumption.
- Economic analysis and cost modeling of carbonyl processes: Conducting comprehensive economic analyses and developing cost models for carbonyl processes helps in identifying cost-saving opportunities and making informed decisions. This involves evaluating raw material costs, energy consumption, equipment expenses, and market dynamics to determine the overall cost-effectiveness of the process.
- Integration of sustainable practices in carbonyl production: Incorporating sustainable practices in carbonyl processes can lead to long-term cost savings and improved environmental performance. This includes implementing green chemistry principles, utilizing renewable feedstocks, and adopting circular economy approaches to reduce waste and improve resource efficiency.
- Advanced process monitoring and control systems: Implementing advanced process monitoring and control systems in carbonyl production can significantly improve cost-effectiveness. These systems utilize real-time data analytics, machine learning algorithms, and predictive maintenance techniques to optimize process performance, reduce downtime, and minimize operational costs.
- Supply chain optimization for carbonyl processes: Optimizing the supply chain for carbonyl processes can lead to substantial cost savings. This involves streamlining raw material procurement, improving inventory management, enhancing logistics, and implementing just-in-time production strategies to reduce overall operational costs and improve efficiency.
02 Economic analysis and modeling of carbonyl processes
Utilizing advanced economic modeling and analysis tools to evaluate the cost-effectiveness of carbonyl processes. This includes assessing raw material costs, energy consumption, equipment expenses, and market demand. Such analyses help in making informed decisions about process improvements and investments to enhance cost-effectiveness.Expand Specific Solutions03 Integration of green chemistry principles
Incorporating green chemistry principles into carbonyl processes to improve sustainability and cost-effectiveness. This involves using renewable feedstocks, developing atom-efficient reactions, and reducing or eliminating the use of hazardous substances. Such approaches can lead to reduced waste treatment costs and improved environmental compliance.Expand Specific Solutions04 Automation and process control improvements
Implementing advanced automation and process control systems in carbonyl processes to enhance efficiency and reduce operational costs. This includes using real-time monitoring, predictive maintenance, and artificial intelligence-driven optimization to minimize downtime, reduce energy consumption, and improve product quality.Expand Specific Solutions05 Supply chain and logistics optimization
Optimizing the supply chain and logistics associated with carbonyl processes to reduce overall costs. This involves improving raw material sourcing, inventory management, and distribution networks. Efficient supply chain management can lead to reduced storage costs, minimized transportation expenses, and improved responsiveness to market demands.Expand Specific Solutions
Key Industry Players and Competition
The carbonyl process research for cost-effective manufacturing is in a mature stage, with a substantial market size due to its wide industrial applications. The technology's maturity is evident from the involvement of established chemical giants like BASF, Wacker Chemie, and Sumitomo Chemical. These companies, along with specialized firms such as Novomer and Directa Plus, are driving innovation in the field. The competitive landscape is characterized by a mix of large multinational corporations and niche players, each contributing to advancements in carbonyl process efficiency and sustainability. As environmental concerns grow, research is likely focusing on greener manufacturing approaches, potentially opening new market opportunities.
BASF Corp.
Technical Solution: BASF has developed an innovative carbonyl process for cost-effective manufacturing of various chemicals. Their approach involves the use of carbon monoxide and hydrogen in the presence of metal catalysts to produce aldehydes and alcohols. This process, known as oxo synthesis or hydroformylation, allows for the efficient production of important industrial chemicals[1]. BASF has optimized this process to achieve high selectivity and yield, reducing waste and energy consumption. They have also implemented advanced process control systems to ensure consistent product quality and maximize production efficiency[2]. Additionally, BASF has explored the use of renewable feedstocks in their carbonyl processes, aligning with sustainability goals[3].
Strengths: High efficiency, reduced waste, and potential for sustainable production. Weaknesses: Dependence on metal catalysts and potential safety concerns associated with carbon monoxide handling.
Novomer, Inc.
Technical Solution: Novomer has pioneered a novel approach to carbonyl processes, focusing on the conversion of waste carbon dioxide (CO2) into valuable polymers and chemicals. Their proprietary catalyst technology enables the copolymerization of CO2 with epoxides to produce polycarbonates and polyols[4]. This process not only provides a cost-effective manufacturing method but also contributes to carbon capture and utilization. Novomer's technology can produce high-performance materials with up to 50% CO2 content, significantly reducing the carbon footprint of the final products[5]. The company has also developed scalable processes for the production of acrylic acid and acrylate esters using CO2 as a feedstock, offering a more sustainable alternative to traditional petroleum-based routes[6].
Strengths: Utilization of waste CO2, reduced carbon footprint, and production of high-performance materials. Weaknesses: Limited to specific product types and potential challenges in scaling up CO2-based processes.
Innovative Carbonyl Reaction Mechanisms
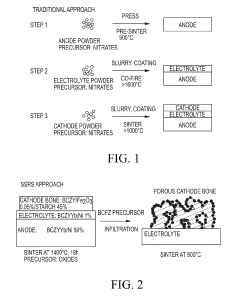
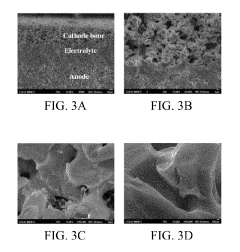
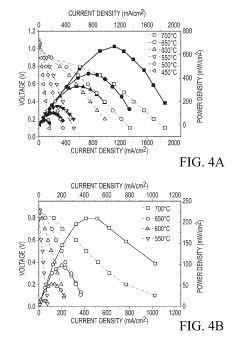
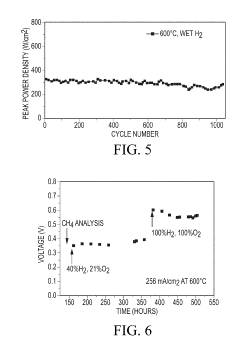
Cost-effective solid state reactive sintering method for protonic ceramic fuel cells
PatentInactiveUS10305116B2
Innovation
- A one-step solid-state reactive sintering (SSRS) method is used to fabricate PCFCs at moderate temperatures, integrating a dense electrolyte, porous anode, and porous cathode with proton conducting ceramic phases, allowing for scalable and cost-effective production of high-performance fuel cells.
Preparation of carbonyl compounds from alcohols
PatentInactiveUS6790997B2
Innovation
- A process utilizing catalytic quantities of osmium compounds in the presence of oxygen as an oxidizing agent in a two-phase solvent system with a pH range of 7 to 14, allowing for high-yield and high-purity production of carbonyl compounds with minimal catalyst usage.
Environmental Impact of Carbonyl Processes
Carbonyl processes, while essential in various manufacturing sectors, have significant environmental implications that require careful consideration. These processes often involve the use of volatile organic compounds (VOCs) and heavy metals, which can lead to air and water pollution if not properly managed.
One of the primary environmental concerns associated with carbonyl processes is the emission of VOCs. These compounds contribute to the formation of ground-level ozone and smog, which can have detrimental effects on human health and ecosystems. Additionally, some VOCs used in carbonyl processes are known carcinogens, posing long-term health risks to workers and surrounding communities.
Water pollution is another critical issue arising from carbonyl processes. Wastewater from these operations often contains high levels of organic compounds and heavy metals, which can contaminate water sources if not adequately treated. This pollution can harm aquatic life and potentially enter the food chain, affecting human health indirectly.
The energy-intensive nature of many carbonyl processes also contributes to their environmental footprint. High energy consumption leads to increased greenhouse gas emissions, particularly when fossil fuels are the primary energy source. This aspect of carbonyl processes directly contributes to climate change and its associated environmental impacts.
Waste generation is a further environmental concern. Carbonyl processes often produce hazardous waste materials that require special handling and disposal. Improper management of these wastes can lead to soil contamination and long-term environmental degradation.
However, it's important to note that the environmental impact of carbonyl processes can be mitigated through various strategies. Implementation of closed-loop systems can significantly reduce emissions and waste. Advanced air pollution control technologies, such as thermal oxidizers and scrubbers, can effectively capture and treat VOC emissions.
Water treatment technologies, including advanced oxidation processes and membrane filtration, can help in reducing water pollution from carbonyl processes. Additionally, the adoption of green chemistry principles in process design can lead to the development of more environmentally friendly carbonyl processes, reducing the overall environmental impact.
Efforts to improve energy efficiency in carbonyl processes can also contribute to reducing their environmental footprint. This can include the use of more efficient equipment, heat recovery systems, and the integration of renewable energy sources where possible.
In conclusion, while carbonyl processes present significant environmental challenges, ongoing research and technological advancements are paving the way for more sustainable manufacturing approaches. Balancing the economic benefits of these processes with their environmental impact remains a key focus for the industry and regulatory bodies alike.
One of the primary environmental concerns associated with carbonyl processes is the emission of VOCs. These compounds contribute to the formation of ground-level ozone and smog, which can have detrimental effects on human health and ecosystems. Additionally, some VOCs used in carbonyl processes are known carcinogens, posing long-term health risks to workers and surrounding communities.
Water pollution is another critical issue arising from carbonyl processes. Wastewater from these operations often contains high levels of organic compounds and heavy metals, which can contaminate water sources if not adequately treated. This pollution can harm aquatic life and potentially enter the food chain, affecting human health indirectly.
The energy-intensive nature of many carbonyl processes also contributes to their environmental footprint. High energy consumption leads to increased greenhouse gas emissions, particularly when fossil fuels are the primary energy source. This aspect of carbonyl processes directly contributes to climate change and its associated environmental impacts.
Waste generation is a further environmental concern. Carbonyl processes often produce hazardous waste materials that require special handling and disposal. Improper management of these wastes can lead to soil contamination and long-term environmental degradation.
However, it's important to note that the environmental impact of carbonyl processes can be mitigated through various strategies. Implementation of closed-loop systems can significantly reduce emissions and waste. Advanced air pollution control technologies, such as thermal oxidizers and scrubbers, can effectively capture and treat VOC emissions.
Water treatment technologies, including advanced oxidation processes and membrane filtration, can help in reducing water pollution from carbonyl processes. Additionally, the adoption of green chemistry principles in process design can lead to the development of more environmentally friendly carbonyl processes, reducing the overall environmental impact.
Efforts to improve energy efficiency in carbonyl processes can also contribute to reducing their environmental footprint. This can include the use of more efficient equipment, heat recovery systems, and the integration of renewable energy sources where possible.
In conclusion, while carbonyl processes present significant environmental challenges, ongoing research and technological advancements are paving the way for more sustainable manufacturing approaches. Balancing the economic benefits of these processes with their environmental impact remains a key focus for the industry and regulatory bodies alike.
Economic Feasibility of Carbonyl Manufacturing
The economic feasibility of carbonyl manufacturing processes is a critical consideration for industries seeking cost-effective production methods. These processes, which involve the use of carbon monoxide as a key reactant, have gained attention due to their potential for efficient and selective synthesis of various organic compounds.
One of the primary advantages of carbonyl manufacturing is its ability to produce high-value chemicals from relatively inexpensive starting materials. The use of carbon monoxide, which can be derived from abundant sources such as natural gas or coal, provides a cost-effective feedstock for the production of a wide range of products, including aldehydes, carboxylic acids, and esters.
The economic viability of carbonyl processes is further enhanced by their high atom economy and selectivity. These characteristics result in reduced waste generation and improved resource utilization, leading to lower overall production costs. Additionally, the mild reaction conditions often employed in carbonyl manufacturing contribute to reduced energy consumption and equipment costs compared to alternative synthetic routes.
However, the economic feasibility of carbonyl manufacturing is not without challenges. The handling and storage of carbon monoxide, a toxic and flammable gas, require specialized equipment and safety measures, which can increase initial capital investments. Furthermore, the cost of catalysts, particularly those containing precious metals, can significantly impact the overall economics of the process.
Despite these challenges, recent advancements in catalyst design and process engineering have improved the economic outlook for carbonyl manufacturing. The development of more efficient and recyclable catalysts has led to increased productivity and reduced operational costs. Moreover, the integration of carbonyl processes into existing chemical production facilities can leverage existing infrastructure, further improving economic viability.
From a market perspective, the demand for products derived from carbonyl processes continues to grow, particularly in industries such as pharmaceuticals, agrochemicals, and specialty chemicals. This expanding market provides a strong economic incentive for the adoption and optimization of carbonyl manufacturing techniques.
In conclusion, the economic feasibility of carbonyl manufacturing processes is generally favorable, driven by their ability to produce valuable chemicals from low-cost feedstocks, high selectivity, and improving process efficiencies. While challenges related to safety and catalyst costs exist, ongoing research and development efforts are continuously enhancing the economic attractiveness of these processes, positioning them as promising approaches for cost-effective chemical production in various industrial sectors.
One of the primary advantages of carbonyl manufacturing is its ability to produce high-value chemicals from relatively inexpensive starting materials. The use of carbon monoxide, which can be derived from abundant sources such as natural gas or coal, provides a cost-effective feedstock for the production of a wide range of products, including aldehydes, carboxylic acids, and esters.
The economic viability of carbonyl processes is further enhanced by their high atom economy and selectivity. These characteristics result in reduced waste generation and improved resource utilization, leading to lower overall production costs. Additionally, the mild reaction conditions often employed in carbonyl manufacturing contribute to reduced energy consumption and equipment costs compared to alternative synthetic routes.
However, the economic feasibility of carbonyl manufacturing is not without challenges. The handling and storage of carbon monoxide, a toxic and flammable gas, require specialized equipment and safety measures, which can increase initial capital investments. Furthermore, the cost of catalysts, particularly those containing precious metals, can significantly impact the overall economics of the process.
Despite these challenges, recent advancements in catalyst design and process engineering have improved the economic outlook for carbonyl manufacturing. The development of more efficient and recyclable catalysts has led to increased productivity and reduced operational costs. Moreover, the integration of carbonyl processes into existing chemical production facilities can leverage existing infrastructure, further improving economic viability.
From a market perspective, the demand for products derived from carbonyl processes continues to grow, particularly in industries such as pharmaceuticals, agrochemicals, and specialty chemicals. This expanding market provides a strong economic incentive for the adoption and optimization of carbonyl manufacturing techniques.
In conclusion, the economic feasibility of carbonyl manufacturing processes is generally favorable, driven by their ability to produce valuable chemicals from low-cost feedstocks, high selectivity, and improving process efficiencies. While challenges related to safety and catalyst costs exist, ongoing research and development efforts are continuously enhancing the economic attractiveness of these processes, positioning them as promising approaches for cost-effective chemical production in various industrial sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!