How to Harness Carbonyl Chemistry for Enhanced Performance?
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Chemistry Background and Objectives
Carbonyl chemistry has been a cornerstone of organic synthesis and materials science for over a century. This versatile functional group, characterized by a carbon-oxygen double bond, plays a crucial role in numerous chemical reactions and industrial processes. The evolution of carbonyl chemistry has been marked by significant breakthroughs in understanding reaction mechanisms, developing new synthetic methodologies, and exploring novel applications across various sectors.
The primary objective of harnessing carbonyl chemistry for enhanced performance is to leverage its unique reactivity and structural properties to create more efficient, sustainable, and high-performing materials and processes. This encompasses a wide range of applications, from pharmaceutical synthesis to advanced materials development and energy storage solutions.
One of the key trends in carbonyl chemistry is the exploration of green chemistry principles. Researchers are focusing on developing environmentally friendly catalysts and reaction conditions that minimize waste and energy consumption while maximizing yield and selectivity. This aligns with the growing global emphasis on sustainability and responsible resource management in chemical industries.
Another significant trend is the integration of carbonyl chemistry with other emerging fields, such as nanotechnology and materials science. By combining the reactivity of carbonyl compounds with advanced materials, researchers aim to create novel functional materials with enhanced properties, such as self-healing polymers, smart coatings, and responsive drug delivery systems.
The advent of computational chemistry and artificial intelligence has also opened new avenues for carbonyl chemistry research. These tools enable more accurate prediction of reaction outcomes, design of new catalysts, and optimization of reaction conditions, accelerating the discovery and development of innovative carbonyl-based technologies.
In the pharmaceutical industry, carbonyl chemistry continues to be instrumental in drug discovery and synthesis. The focus is on developing more selective and efficient methods for creating complex molecular structures, particularly in the realm of asymmetric synthesis and late-stage functionalization of drug candidates.
As we look towards the future, the objectives for harnessing carbonyl chemistry include developing more atom-economical reactions, exploring novel activation modes for carbonyl compounds, and expanding the scope of carbonyl chemistry in materials science and energy applications. There is also a growing interest in utilizing renewable resources as starting materials for carbonyl-based syntheses, aligning with the principles of circular economy and sustainable chemistry.
The primary objective of harnessing carbonyl chemistry for enhanced performance is to leverage its unique reactivity and structural properties to create more efficient, sustainable, and high-performing materials and processes. This encompasses a wide range of applications, from pharmaceutical synthesis to advanced materials development and energy storage solutions.
One of the key trends in carbonyl chemistry is the exploration of green chemistry principles. Researchers are focusing on developing environmentally friendly catalysts and reaction conditions that minimize waste and energy consumption while maximizing yield and selectivity. This aligns with the growing global emphasis on sustainability and responsible resource management in chemical industries.
Another significant trend is the integration of carbonyl chemistry with other emerging fields, such as nanotechnology and materials science. By combining the reactivity of carbonyl compounds with advanced materials, researchers aim to create novel functional materials with enhanced properties, such as self-healing polymers, smart coatings, and responsive drug delivery systems.
The advent of computational chemistry and artificial intelligence has also opened new avenues for carbonyl chemistry research. These tools enable more accurate prediction of reaction outcomes, design of new catalysts, and optimization of reaction conditions, accelerating the discovery and development of innovative carbonyl-based technologies.
In the pharmaceutical industry, carbonyl chemistry continues to be instrumental in drug discovery and synthesis. The focus is on developing more selective and efficient methods for creating complex molecular structures, particularly in the realm of asymmetric synthesis and late-stage functionalization of drug candidates.
As we look towards the future, the objectives for harnessing carbonyl chemistry include developing more atom-economical reactions, exploring novel activation modes for carbonyl compounds, and expanding the scope of carbonyl chemistry in materials science and energy applications. There is also a growing interest in utilizing renewable resources as starting materials for carbonyl-based syntheses, aligning with the principles of circular economy and sustainable chemistry.
Market Demand for Carbonyl-Based Solutions
The market demand for carbonyl-based solutions has been steadily growing across various industries, driven by the unique properties and versatile applications of carbonyl compounds. In the pharmaceutical sector, carbonyl chemistry plays a crucial role in drug discovery and development, with aldol reactions and Mannich reactions being widely used in the synthesis of complex drug molecules. This has led to an increased demand for carbonyl-based intermediates and catalysts in the pharmaceutical industry.
The food and beverage industry has also shown significant interest in carbonyl chemistry, particularly in the development of flavors and fragrances. Aldehydes and ketones are essential components in creating a wide range of aromas and tastes, driving the demand for carbonyl-based solutions in this sector. Additionally, the growing consumer preference for natural and clean-label products has spurred research into bio-based carbonyl compounds, further expanding market opportunities.
In the materials science field, carbonyl chemistry has found applications in the development of advanced polymers and coatings. The ability of carbonyl groups to participate in various reactions has led to the creation of high-performance materials with enhanced properties such as improved adhesion, durability, and chemical resistance. This has resulted in a growing demand for carbonyl-based monomers and crosslinking agents in the polymer and coatings industries.
The agrochemical sector has also witnessed an increased demand for carbonyl-based solutions, particularly in the development of more effective and environmentally friendly pesticides and herbicides. Carbonyl compounds serve as key building blocks in the synthesis of many agrochemicals, driving research into novel formulations with improved efficacy and reduced environmental impact.
Environmental concerns and sustainability initiatives have further fueled the demand for carbonyl-based solutions in green chemistry applications. The development of bio-based carbonyl compounds and their use in sustainable chemical processes has gained significant traction, with industries seeking alternatives to petroleum-based chemicals.
The global market for carbonyl compounds is projected to experience substantial growth in the coming years, with Asia-Pacific emerging as a key region for market expansion. Factors such as rapid industrialization, increasing investments in research and development, and growing demand from end-use industries are expected to drive this growth. However, challenges such as stringent regulations on volatile organic compounds (VOCs) and the need for more efficient and sustainable production methods may impact market dynamics.
The food and beverage industry has also shown significant interest in carbonyl chemistry, particularly in the development of flavors and fragrances. Aldehydes and ketones are essential components in creating a wide range of aromas and tastes, driving the demand for carbonyl-based solutions in this sector. Additionally, the growing consumer preference for natural and clean-label products has spurred research into bio-based carbonyl compounds, further expanding market opportunities.
In the materials science field, carbonyl chemistry has found applications in the development of advanced polymers and coatings. The ability of carbonyl groups to participate in various reactions has led to the creation of high-performance materials with enhanced properties such as improved adhesion, durability, and chemical resistance. This has resulted in a growing demand for carbonyl-based monomers and crosslinking agents in the polymer and coatings industries.
The agrochemical sector has also witnessed an increased demand for carbonyl-based solutions, particularly in the development of more effective and environmentally friendly pesticides and herbicides. Carbonyl compounds serve as key building blocks in the synthesis of many agrochemicals, driving research into novel formulations with improved efficacy and reduced environmental impact.
Environmental concerns and sustainability initiatives have further fueled the demand for carbonyl-based solutions in green chemistry applications. The development of bio-based carbonyl compounds and their use in sustainable chemical processes has gained significant traction, with industries seeking alternatives to petroleum-based chemicals.
The global market for carbonyl compounds is projected to experience substantial growth in the coming years, with Asia-Pacific emerging as a key region for market expansion. Factors such as rapid industrialization, increasing investments in research and development, and growing demand from end-use industries are expected to drive this growth. However, challenges such as stringent regulations on volatile organic compounds (VOCs) and the need for more efficient and sustainable production methods may impact market dynamics.
Current Challenges in Carbonyl Chemistry
Carbonyl chemistry, a cornerstone of organic synthesis, faces several significant challenges that hinder its full potential in enhancing performance across various applications. One of the primary obstacles is the control of selectivity in carbonyl reactions. While carbonyl compounds are highly reactive, achieving precise control over the stereochemistry and regioselectivity of reactions remains a persistent challenge. This is particularly evident in complex molecule synthesis, where multiple carbonyl groups may be present, leading to unwanted side reactions and reduced yields.
Another critical challenge lies in the development of more efficient and sustainable catalytic systems for carbonyl transformations. Traditional methods often rely on stoichiometric amounts of reagents or harsh reaction conditions, which are not environmentally friendly and can be economically unfavorable for large-scale production. The search for catalysts that can operate under milder conditions, with lower catalyst loadings, and improved atom economy is ongoing but faces significant hurdles in terms of catalyst design and optimization.
The stability of carbonyl compounds and their intermediates poses another challenge, especially in industrial applications. Many carbonyl-containing molecules are sensitive to heat, light, or pH changes, which can lead to degradation or unwanted side reactions during storage or processing. This instability can result in reduced shelf life of products and complications in manufacturing processes, necessitating the development of stabilization strategies or alternative formulations.
Furthermore, the scalability of carbonyl chemistry processes presents a significant challenge when transitioning from laboratory-scale reactions to industrial production. Issues such as heat transfer, mixing efficiency, and reaction kinetics can differ dramatically at larger scales, often requiring extensive process optimization and sometimes complete redesign of synthetic routes.
In the realm of biomass conversion and utilization, harnessing carbonyl chemistry for the efficient transformation of renewable resources into value-added chemicals and materials remains a complex challenge. The diverse and often inconsistent nature of biomass feedstocks complicates the development of robust and widely applicable carbonyl-based conversion processes.
Lastly, the integration of carbonyl chemistry with emerging technologies, such as flow chemistry and artificial intelligence for reaction prediction and optimization, presents both opportunities and challenges. While these technologies offer the potential for significant advancements, their successful implementation requires overcoming barriers in data availability, model accuracy, and the development of suitable hardware for continuous-flow carbonyl transformations.
Addressing these challenges is crucial for unlocking the full potential of carbonyl chemistry in enhancing performance across various fields, from pharmaceuticals and materials science to energy and environmental applications. Overcoming these obstacles will require interdisciplinary approaches, combining advances in synthetic methodology, catalysis, process engineering, and computational chemistry.
Another critical challenge lies in the development of more efficient and sustainable catalytic systems for carbonyl transformations. Traditional methods often rely on stoichiometric amounts of reagents or harsh reaction conditions, which are not environmentally friendly and can be economically unfavorable for large-scale production. The search for catalysts that can operate under milder conditions, with lower catalyst loadings, and improved atom economy is ongoing but faces significant hurdles in terms of catalyst design and optimization.
The stability of carbonyl compounds and their intermediates poses another challenge, especially in industrial applications. Many carbonyl-containing molecules are sensitive to heat, light, or pH changes, which can lead to degradation or unwanted side reactions during storage or processing. This instability can result in reduced shelf life of products and complications in manufacturing processes, necessitating the development of stabilization strategies or alternative formulations.
Furthermore, the scalability of carbonyl chemistry processes presents a significant challenge when transitioning from laboratory-scale reactions to industrial production. Issues such as heat transfer, mixing efficiency, and reaction kinetics can differ dramatically at larger scales, often requiring extensive process optimization and sometimes complete redesign of synthetic routes.
In the realm of biomass conversion and utilization, harnessing carbonyl chemistry for the efficient transformation of renewable resources into value-added chemicals and materials remains a complex challenge. The diverse and often inconsistent nature of biomass feedstocks complicates the development of robust and widely applicable carbonyl-based conversion processes.
Lastly, the integration of carbonyl chemistry with emerging technologies, such as flow chemistry and artificial intelligence for reaction prediction and optimization, presents both opportunities and challenges. While these technologies offer the potential for significant advancements, their successful implementation requires overcoming barriers in data availability, model accuracy, and the development of suitable hardware for continuous-flow carbonyl transformations.
Addressing these challenges is crucial for unlocking the full potential of carbonyl chemistry in enhancing performance across various fields, from pharmaceuticals and materials science to energy and environmental applications. Overcoming these obstacles will require interdisciplinary approaches, combining advances in synthetic methodology, catalysis, process engineering, and computational chemistry.
Existing Carbonyl Enhancement Strategies
01 Carbonyl compound synthesis and reactions
This category focuses on the synthesis and reactions of carbonyl compounds, including aldehydes and ketones. It covers various methods for producing these compounds and their subsequent transformations, such as oxidation, reduction, and condensation reactions. The performance of these reactions is crucial in organic synthesis and industrial applications.- Carbonyl compound synthesis and reactions: This category focuses on the synthesis and reactions of carbonyl compounds, including aldehydes and ketones. It covers various methods for preparing these compounds and their subsequent transformations, such as oxidation, reduction, and condensation reactions. The performance of these reactions is often evaluated in terms of yield, selectivity, and reaction conditions.
- Carbonyl chemistry in organic synthesis: This area explores the use of carbonyl compounds as key intermediates in organic synthesis. It includes the application of carbonyl chemistry in the construction of complex molecules, natural product synthesis, and the development of new synthetic methodologies. The performance is assessed based on the efficiency of carbon-carbon bond formation and functional group transformations.
- Catalytic processes in carbonyl chemistry: This category covers the development and application of catalysts to improve the performance of carbonyl chemistry reactions. It includes homogeneous and heterogeneous catalysis, organocatalysis, and biocatalysis. The focus is on enhancing reaction rates, selectivity, and sustainability of carbonyl transformations.
- Analytical methods for carbonyl compounds: This area deals with the development and optimization of analytical techniques for the detection, quantification, and characterization of carbonyl compounds. It includes spectroscopic methods, chromatography, and sensor technologies. The performance is evaluated based on sensitivity, selectivity, and reliability of the analytical methods.
- Industrial applications of carbonyl chemistry: This category focuses on the practical applications of carbonyl chemistry in various industries, including pharmaceuticals, materials science, and chemical manufacturing. It covers process optimization, scale-up strategies, and the development of new products based on carbonyl chemistry. Performance is measured in terms of product quality, process efficiency, and economic viability.
02 Carbonyl chemistry in material science
Carbonyl chemistry plays a significant role in material science, particularly in the development of polymers, coatings, and advanced materials. This area explores the use of carbonyl compounds in creating new materials with specific properties, such as improved durability, flexibility, or reactivity.Expand Specific Solutions03 Analytical methods for carbonyl compounds
This category covers various analytical techniques used to detect, quantify, and characterize carbonyl compounds. It includes spectroscopic methods, chromatography, and other advanced analytical tools that are essential for monitoring carbonyl chemistry performance in research and industrial settings.Expand Specific Solutions04 Carbonyl chemistry in pharmaceutical applications
Carbonyl chemistry is crucial in pharmaceutical research and development. This area focuses on the use of carbonyl compounds in drug synthesis, their role in drug metabolism, and the development of carbonyl-based drug delivery systems. The performance of carbonyl reactions is critical for the efficacy and safety of pharmaceutical products.Expand Specific Solutions05 Green chemistry approaches in carbonyl reactions
This category explores environmentally friendly approaches to carbonyl chemistry. It includes the development of sustainable catalysts, solvent-free reactions, and other green chemistry techniques that improve the efficiency and reduce the environmental impact of carbonyl reactions while maintaining or enhancing their performance.Expand Specific Solutions
Key Players in Carbonyl Chemistry Research
The carbonyl chemistry market is in a growth phase, driven by increasing demand across various industries. The global market size is expanding, with significant potential in pharmaceuticals, materials science, and industrial applications. Technological maturity varies across different applications, but overall, the field is advancing rapidly. Key players like BASF Corp., Celanese International Corp., and Sumitomo Chemical Co., Ltd. are leading innovation in this space. Chinese companies such as China Petroleum & Chemical Corp. and PetroChina Co., Ltd. are also making significant strides. Research institutions like the Council of Scientific & Industrial Research and universities are contributing to fundamental advancements. The competitive landscape is diverse, with both established chemical giants and specialized firms like Directa Plus SpA focusing on niche applications of carbonyl chemistry.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has made significant strides in harnessing carbonyl chemistry for enhanced performance across various industries. They have developed innovative cross-coupling methodologies involving carbonyl compounds, enabling the efficient synthesis of complex molecules for pharmaceutical and agrochemical applications[1]. Their approach utilizes transition metal catalysts with carefully designed ligands to achieve high selectivity and functional group tolerance. Sumitomo has also pioneered the use of carbonyl chemistry in polymer science, developing novel monomers and polymerization techniques for high-performance materials[3]. Additionally, they have explored the application of carbonyl chemistry in electronic materials, particularly in the development of organic light-emitting diodes (OLEDs) and organic photovoltaics[5]. This research has led to improvements in device efficiency and stability.
Strengths: Diverse applications from pharmaceuticals to electronic materials; strong focus on practical industrial applications. Weaknesses: Some technologies may require specialized catalysts or precursors, potentially limiting widespread adoption.
BASF Corp.
Technical Solution: BASF has developed innovative carbonyl chemistry techniques to enhance performance in various applications. They have pioneered the use of metal-organic frameworks (MOFs) for selective carbonyl capture and conversion[1]. Their approach involves tailoring the pore size and functionality of MOFs to selectively adsorb and react with specific carbonyl compounds. This allows for more efficient and targeted chemical transformations. BASF has also made advancements in asymmetric hydrogenation of carbonyls using chiral transition metal catalysts, achieving high enantioselectivity for pharmaceutical and fine chemical synthesis[3]. Additionally, they have developed novel organocatalytic methods for carbonyl activation, enabling greener and more atom-economical transformations[5].
Strengths: Wide range of applications from fine chemicals to industrial processes; high selectivity and efficiency. Weaknesses: Some methods may require specialized catalysts or reaction conditions, potentially limiting scalability.
Innovative Approaches in Carbonyl Catalysis
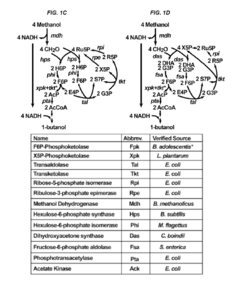
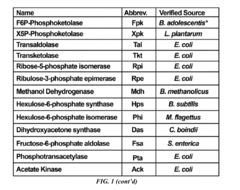
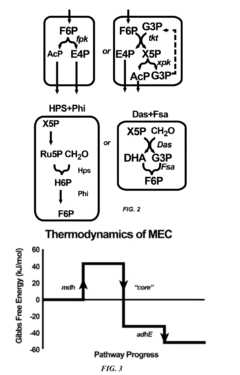
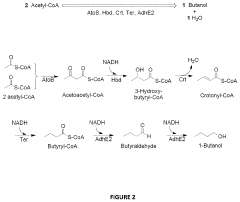
Recombinant microorganisms having a methanol elongation cycle (MEC)
PatentActiveUS20160060635A1
Innovation
- A recombinant microorganism with a metabolic pathway that converts methanol or formaldehyde to acetyl phosphate using enzymes such as phosphoketolase, hexulose-6-phosphate synthase, and transketolase, bypassing pyruvate oxidation to achieve stoichiometric conversion without carbon loss.
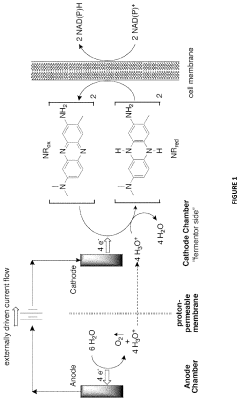
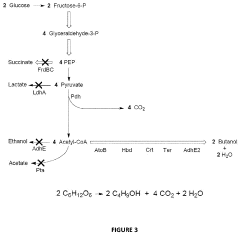
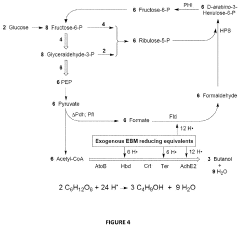
Methods and Systems for 1-Butanol Production
PatentActiveUS20200325498A1
Innovation
- A novel combination of metabolic engineering and electrochemical bioreactor technology is employed, where an electrochemical bioreactor module provides reducing equivalents to engineered metabolic pathways in a host cell, allowing for the conversion of glucose to 1-butanol with 100% carbon efficiency by capturing carbon as formate and recycling it back into the glycolytic pathway, avoiding CO2 production.
Green Chemistry Aspects of Carbonyl Reactions
Green chemistry principles have become increasingly important in the field of carbonyl chemistry, offering innovative approaches to enhance performance while minimizing environmental impact. The application of these principles to carbonyl reactions has led to significant advancements in sustainable synthesis and process optimization.
One key aspect of green carbonyl chemistry is the development of atom-efficient reactions. Traditional carbonyl transformations often generate substantial waste, but recent research has focused on designing reactions that maximize the incorporation of reactants into the final product. This approach not only reduces waste but also improves overall reaction efficiency.
Solvent selection plays a crucial role in green carbonyl chemistry. Water-based reactions have gained prominence due to their environmental friendliness and cost-effectiveness. Additionally, the use of bio-derived solvents and ionic liquids has shown promise in replacing conventional organic solvents, offering improved safety profiles and potential for recycling.
Catalysis has emerged as a powerful tool in green carbonyl chemistry. Biocatalysts, such as enzymes, have demonstrated remarkable selectivity and efficiency in carbonyl transformations under mild conditions. Furthermore, the development of heterogeneous catalysts has facilitated easier product separation and catalyst recycling, aligning with green chemistry principles.
Energy efficiency is another critical consideration in green carbonyl reactions. The implementation of microwave-assisted and photocatalytic processes has enabled faster reaction times and reduced energy consumption. These methods often allow for milder reaction conditions, further enhancing their green credentials.
The concept of renewable feedstocks has gained traction in carbonyl chemistry. Researchers are exploring the use of biomass-derived platform chemicals as starting materials for various carbonyl compounds, reducing reliance on petroleum-based resources. This shift towards bio-based precursors contributes to the development of more sustainable chemical processes.
Green metrics have been developed to assess and quantify the environmental impact of carbonyl reactions. Tools such as E-factor (Environmental factor) and atom economy calculations help researchers optimize reaction conditions and compare different synthetic routes based on their sustainability profiles.
In conclusion, the integration of green chemistry principles into carbonyl reactions has opened up new avenues for enhanced performance and sustainability. By focusing on atom efficiency, environmentally benign solvents, catalysis, energy efficiency, renewable feedstocks, and green metrics, researchers are paving the way for more sustainable and efficient carbonyl chemistry processes.
One key aspect of green carbonyl chemistry is the development of atom-efficient reactions. Traditional carbonyl transformations often generate substantial waste, but recent research has focused on designing reactions that maximize the incorporation of reactants into the final product. This approach not only reduces waste but also improves overall reaction efficiency.
Solvent selection plays a crucial role in green carbonyl chemistry. Water-based reactions have gained prominence due to their environmental friendliness and cost-effectiveness. Additionally, the use of bio-derived solvents and ionic liquids has shown promise in replacing conventional organic solvents, offering improved safety profiles and potential for recycling.
Catalysis has emerged as a powerful tool in green carbonyl chemistry. Biocatalysts, such as enzymes, have demonstrated remarkable selectivity and efficiency in carbonyl transformations under mild conditions. Furthermore, the development of heterogeneous catalysts has facilitated easier product separation and catalyst recycling, aligning with green chemistry principles.
Energy efficiency is another critical consideration in green carbonyl reactions. The implementation of microwave-assisted and photocatalytic processes has enabled faster reaction times and reduced energy consumption. These methods often allow for milder reaction conditions, further enhancing their green credentials.
The concept of renewable feedstocks has gained traction in carbonyl chemistry. Researchers are exploring the use of biomass-derived platform chemicals as starting materials for various carbonyl compounds, reducing reliance on petroleum-based resources. This shift towards bio-based precursors contributes to the development of more sustainable chemical processes.
Green metrics have been developed to assess and quantify the environmental impact of carbonyl reactions. Tools such as E-factor (Environmental factor) and atom economy calculations help researchers optimize reaction conditions and compare different synthetic routes based on their sustainability profiles.
In conclusion, the integration of green chemistry principles into carbonyl reactions has opened up new avenues for enhanced performance and sustainability. By focusing on atom efficiency, environmentally benign solvents, catalysis, energy efficiency, renewable feedstocks, and green metrics, researchers are paving the way for more sustainable and efficient carbonyl chemistry processes.
Industrial Applications of Enhanced Carbonyl Chemistry
Carbonyl chemistry has emerged as a powerful tool in various industrial applications, offering enhanced performance and efficiency across multiple sectors. In the pharmaceutical industry, carbonyl compounds play a crucial role in drug synthesis and development. The ability to manipulate carbonyl groups allows for the creation of complex molecular structures, leading to more effective and targeted medications. For instance, the use of aldol condensation reactions in the synthesis of anti-inflammatory drugs has significantly improved their efficacy and reduced side effects.
In the field of materials science, carbonyl chemistry has revolutionized the development of high-performance polymers and composites. The incorporation of carbonyl groups into polymer chains enhances their mechanical properties, thermal stability, and chemical resistance. This has led to the production of advanced materials used in aerospace, automotive, and electronics industries. For example, polyimides, which contain carbonyl groups in their backbone, are widely used in flexible printed circuit boards due to their excellent thermal and electrical properties.
The food and beverage industry has also benefited from enhanced carbonyl chemistry. Carbonyl compounds are responsible for many flavors and aromas in food products. By harnessing carbonyl chemistry, food scientists have developed new flavor enhancers and preservatives that improve taste, extend shelf life, and maintain food quality. Additionally, carbonyl-based reactions, such as the Maillard reaction, are utilized to create desirable flavors and colors in processed foods.
In the energy sector, carbonyl chemistry has contributed to the development of more efficient and environmentally friendly fuel additives. Oxygenated compounds containing carbonyl groups, such as ethers and esters, are used to improve fuel combustion and reduce emissions. These additives enhance engine performance, increase fuel economy, and help meet stringent environmental regulations.
The textile industry has also embraced carbonyl chemistry to enhance fabric properties. Carbonyl-containing compounds are used in the production of wrinkle-resistant and flame-retardant fabrics. These treatments improve the durability and functionality of textiles, making them more suitable for specialized applications in protective clothing and high-performance sportswear.
Furthermore, carbonyl chemistry has found applications in the field of environmental remediation. Carbonyl-based adsorbents and catalysts are employed in water and air purification systems to remove pollutants and contaminants. These materials demonstrate high selectivity and efficiency in capturing harmful substances, contributing to cleaner and safer environments.
In the field of materials science, carbonyl chemistry has revolutionized the development of high-performance polymers and composites. The incorporation of carbonyl groups into polymer chains enhances their mechanical properties, thermal stability, and chemical resistance. This has led to the production of advanced materials used in aerospace, automotive, and electronics industries. For example, polyimides, which contain carbonyl groups in their backbone, are widely used in flexible printed circuit boards due to their excellent thermal and electrical properties.
The food and beverage industry has also benefited from enhanced carbonyl chemistry. Carbonyl compounds are responsible for many flavors and aromas in food products. By harnessing carbonyl chemistry, food scientists have developed new flavor enhancers and preservatives that improve taste, extend shelf life, and maintain food quality. Additionally, carbonyl-based reactions, such as the Maillard reaction, are utilized to create desirable flavors and colors in processed foods.
In the energy sector, carbonyl chemistry has contributed to the development of more efficient and environmentally friendly fuel additives. Oxygenated compounds containing carbonyl groups, such as ethers and esters, are used to improve fuel combustion and reduce emissions. These additives enhance engine performance, increase fuel economy, and help meet stringent environmental regulations.
The textile industry has also embraced carbonyl chemistry to enhance fabric properties. Carbonyl-containing compounds are used in the production of wrinkle-resistant and flame-retardant fabrics. These treatments improve the durability and functionality of textiles, making them more suitable for specialized applications in protective clothing and high-performance sportswear.
Furthermore, carbonyl chemistry has found applications in the field of environmental remediation. Carbonyl-based adsorbents and catalysts are employed in water and air purification systems to remove pollutants and contaminants. These materials demonstrate high selectivity and efficiency in capturing harmful substances, contributing to cleaner and safer environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!