How to Integrate Carbonyl Compounds in Battery Technologies?
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Compounds in Battery Tech: Background and Objectives
The integration of carbonyl compounds in battery technologies represents a significant frontier in energy storage research. This field has evolved from early explorations of organic materials in batteries to the current focus on carbonyl-based compounds as promising candidates for next-generation energy storage solutions. The trajectory of this technology is marked by a growing recognition of the potential of organic materials to address limitations in traditional inorganic battery systems.
Historically, battery technology has been dominated by inorganic materials, particularly lithium-ion chemistries. However, the increasing demand for more sustainable, cost-effective, and high-performance energy storage solutions has driven researchers to explore alternative materials. Carbonyl compounds, with their diverse structures and tunable properties, have emerged as a compelling area of investigation.
The development of carbonyl-based battery technologies can be traced back to the early 2000s when researchers began to seriously consider organic materials for electrochemical energy storage. Initial studies focused on simple quinone structures, which demonstrated the feasibility of using carbonyl groups as redox-active centers. As the field progressed, more complex carbonyl compounds were synthesized and tested, leading to improvements in capacity, cycling stability, and rate capability.
The primary objective of integrating carbonyl compounds in battery technologies is to harness their unique electrochemical properties to create high-performance, sustainable energy storage devices. Specifically, researchers aim to leverage the reversible redox reactions of carbonyl groups to achieve high capacity, rapid charge-discharge capabilities, and long cycle life. Additionally, the use of organic materials addresses concerns about the scarcity and environmental impact of traditional inorganic battery components.
Key technical goals in this field include enhancing the stability of carbonyl compounds during repeated charge-discharge cycles, increasing their energy density to compete with state-of-the-art lithium-ion batteries, and improving their conductivity to facilitate faster electron transfer. Researchers are also focused on developing scalable synthesis methods and exploring ways to integrate these materials into practical battery designs.
The evolution of carbonyl-based battery technology is closely tied to advancements in organic synthesis, electrochemistry, and materials science. As these fields progress, new carbonyl compounds with tailored properties are being developed, pushing the boundaries of what is possible in organic energy storage. The interdisciplinary nature of this research area promises continued innovation and the potential for breakthrough technologies in the coming years.
Historically, battery technology has been dominated by inorganic materials, particularly lithium-ion chemistries. However, the increasing demand for more sustainable, cost-effective, and high-performance energy storage solutions has driven researchers to explore alternative materials. Carbonyl compounds, with their diverse structures and tunable properties, have emerged as a compelling area of investigation.
The development of carbonyl-based battery technologies can be traced back to the early 2000s when researchers began to seriously consider organic materials for electrochemical energy storage. Initial studies focused on simple quinone structures, which demonstrated the feasibility of using carbonyl groups as redox-active centers. As the field progressed, more complex carbonyl compounds were synthesized and tested, leading to improvements in capacity, cycling stability, and rate capability.
The primary objective of integrating carbonyl compounds in battery technologies is to harness their unique electrochemical properties to create high-performance, sustainable energy storage devices. Specifically, researchers aim to leverage the reversible redox reactions of carbonyl groups to achieve high capacity, rapid charge-discharge capabilities, and long cycle life. Additionally, the use of organic materials addresses concerns about the scarcity and environmental impact of traditional inorganic battery components.
Key technical goals in this field include enhancing the stability of carbonyl compounds during repeated charge-discharge cycles, increasing their energy density to compete with state-of-the-art lithium-ion batteries, and improving their conductivity to facilitate faster electron transfer. Researchers are also focused on developing scalable synthesis methods and exploring ways to integrate these materials into practical battery designs.
The evolution of carbonyl-based battery technology is closely tied to advancements in organic synthesis, electrochemistry, and materials science. As these fields progress, new carbonyl compounds with tailored properties are being developed, pushing the boundaries of what is possible in organic energy storage. The interdisciplinary nature of this research area promises continued innovation and the potential for breakthrough technologies in the coming years.
Market Analysis for Carbonyl-Based Battery Solutions
The market for carbonyl-based battery solutions is experiencing significant growth, driven by the increasing demand for high-performance energy storage systems across various industries. As the global push for renewable energy and electrification intensifies, the need for advanced battery technologies has become more pressing. Carbonyl compounds, with their unique electrochemical properties, are emerging as promising candidates for next-generation battery systems.
The automotive sector represents a major market opportunity for carbonyl-based batteries. With the rapid expansion of electric vehicle (EV) production, there is a growing demand for batteries that offer higher energy density, faster charging capabilities, and improved safety profiles. Carbonyl-based batteries have the potential to address these requirements, making them attractive to EV manufacturers seeking to enhance their product offerings and gain a competitive edge in the market.
In the consumer electronics industry, the trend towards more powerful and longer-lasting devices is creating a favorable environment for carbonyl-based battery solutions. Smartphones, laptops, and wearable devices could benefit from the improved energy density and cycle life that carbonyl compounds can provide. This market segment is particularly sensitive to advancements in battery technology, as consumers increasingly prioritize device longevity and quick charging features.
The renewable energy sector presents another significant market opportunity for carbonyl-based batteries. As wind and solar power generation continues to grow, the need for efficient and cost-effective energy storage solutions becomes more critical. Carbonyl-based batteries could play a crucial role in grid stabilization and energy management, offering utilities and renewable energy providers a viable alternative to traditional lithium-ion batteries.
Industrial applications, including backup power systems and material handling equipment, are also potential markets for carbonyl-based battery technologies. These sectors require robust and reliable energy storage solutions that can withstand harsh operating conditions and provide consistent performance over extended periods.
The market potential for carbonyl-based batteries is further enhanced by the growing emphasis on sustainability and environmental responsibility. As companies and governments worldwide seek to reduce their carbon footprint, there is increasing interest in battery technologies that offer improved recyclability and reduced environmental impact. Carbonyl compounds, being organic materials, may provide advantages in terms of sustainability compared to traditional inorganic battery chemistries.
However, the market adoption of carbonyl-based battery solutions faces several challenges. These include the need for further research and development to optimize performance, scale-up manufacturing processes, and address any potential safety concerns. Additionally, the established market presence of lithium-ion batteries and the significant investments already made in their production infrastructure could pose barriers to entry for new battery chemistries.
The automotive sector represents a major market opportunity for carbonyl-based batteries. With the rapid expansion of electric vehicle (EV) production, there is a growing demand for batteries that offer higher energy density, faster charging capabilities, and improved safety profiles. Carbonyl-based batteries have the potential to address these requirements, making them attractive to EV manufacturers seeking to enhance their product offerings and gain a competitive edge in the market.
In the consumer electronics industry, the trend towards more powerful and longer-lasting devices is creating a favorable environment for carbonyl-based battery solutions. Smartphones, laptops, and wearable devices could benefit from the improved energy density and cycle life that carbonyl compounds can provide. This market segment is particularly sensitive to advancements in battery technology, as consumers increasingly prioritize device longevity and quick charging features.
The renewable energy sector presents another significant market opportunity for carbonyl-based batteries. As wind and solar power generation continues to grow, the need for efficient and cost-effective energy storage solutions becomes more critical. Carbonyl-based batteries could play a crucial role in grid stabilization and energy management, offering utilities and renewable energy providers a viable alternative to traditional lithium-ion batteries.
Industrial applications, including backup power systems and material handling equipment, are also potential markets for carbonyl-based battery technologies. These sectors require robust and reliable energy storage solutions that can withstand harsh operating conditions and provide consistent performance over extended periods.
The market potential for carbonyl-based batteries is further enhanced by the growing emphasis on sustainability and environmental responsibility. As companies and governments worldwide seek to reduce their carbon footprint, there is increasing interest in battery technologies that offer improved recyclability and reduced environmental impact. Carbonyl compounds, being organic materials, may provide advantages in terms of sustainability compared to traditional inorganic battery chemistries.
However, the market adoption of carbonyl-based battery solutions faces several challenges. These include the need for further research and development to optimize performance, scale-up manufacturing processes, and address any potential safety concerns. Additionally, the established market presence of lithium-ion batteries and the significant investments already made in their production infrastructure could pose barriers to entry for new battery chemistries.
Current Challenges in Carbonyl Compound Integration
The integration of carbonyl compounds in battery technologies faces several significant challenges that hinder widespread adoption and commercialization. One of the primary obstacles is the inherent instability of carbonyl compounds, particularly in the presence of electrolytes commonly used in batteries. This instability can lead to unwanted side reactions, resulting in capacity fading and reduced cycle life of the battery.
Another major challenge is the solubility of carbonyl compounds in organic electrolytes. While this property can be advantageous for certain applications, it often leads to the dissolution of active materials from the electrode, causing loss of capacity and potential safety issues. Researchers are actively exploring ways to mitigate this problem, such as developing novel electrolyte formulations or employing polymer coatings to encapsulate the active materials.
The electronic conductivity of carbonyl compounds presents an additional hurdle. Many organic carbonyl compounds exhibit poor electronic conductivity, which can limit the rate capability and overall performance of the battery. To address this issue, scientists are investigating various approaches, including the incorporation of conductive additives and the design of carbonyl-based materials with improved intrinsic conductivity.
Voltage hysteresis is another significant challenge in carbonyl-based battery systems. The difference between charge and discharge voltages can lead to energy inefficiencies and reduced overall battery performance. Overcoming this issue requires a deeper understanding of the redox mechanisms involved and the development of strategies to minimize voltage gaps.
The scalability of carbonyl compound synthesis and integration into battery manufacturing processes also poses challenges. Many promising carbonyl-based materials have been demonstrated at the laboratory scale, but translating these results to large-scale production remains difficult. Developing cost-effective and environmentally friendly synthesis methods that can be scaled up for industrial production is crucial for the widespread adoption of carbonyl-based battery technologies.
Furthermore, the long-term stability and safety of carbonyl compounds in battery applications need to be thoroughly addressed. Concerns about potential degradation products and their impact on battery performance and safety must be carefully evaluated and mitigated before these materials can be widely implemented in commercial battery systems.
Lastly, optimizing the energy density of carbonyl-based batteries remains a challenge. While some carbonyl compounds offer high theoretical capacities, achieving practical energy densities that can compete with state-of-the-art lithium-ion batteries requires further research and development. This includes exploring new molecular designs, optimizing electrode architectures, and developing advanced electrolyte systems tailored for carbonyl-based chemistries.
Another major challenge is the solubility of carbonyl compounds in organic electrolytes. While this property can be advantageous for certain applications, it often leads to the dissolution of active materials from the electrode, causing loss of capacity and potential safety issues. Researchers are actively exploring ways to mitigate this problem, such as developing novel electrolyte formulations or employing polymer coatings to encapsulate the active materials.
The electronic conductivity of carbonyl compounds presents an additional hurdle. Many organic carbonyl compounds exhibit poor electronic conductivity, which can limit the rate capability and overall performance of the battery. To address this issue, scientists are investigating various approaches, including the incorporation of conductive additives and the design of carbonyl-based materials with improved intrinsic conductivity.
Voltage hysteresis is another significant challenge in carbonyl-based battery systems. The difference between charge and discharge voltages can lead to energy inefficiencies and reduced overall battery performance. Overcoming this issue requires a deeper understanding of the redox mechanisms involved and the development of strategies to minimize voltage gaps.
The scalability of carbonyl compound synthesis and integration into battery manufacturing processes also poses challenges. Many promising carbonyl-based materials have been demonstrated at the laboratory scale, but translating these results to large-scale production remains difficult. Developing cost-effective and environmentally friendly synthesis methods that can be scaled up for industrial production is crucial for the widespread adoption of carbonyl-based battery technologies.
Furthermore, the long-term stability and safety of carbonyl compounds in battery applications need to be thoroughly addressed. Concerns about potential degradation products and their impact on battery performance and safety must be carefully evaluated and mitigated before these materials can be widely implemented in commercial battery systems.
Lastly, optimizing the energy density of carbonyl-based batteries remains a challenge. While some carbonyl compounds offer high theoretical capacities, achieving practical energy densities that can compete with state-of-the-art lithium-ion batteries requires further research and development. This includes exploring new molecular designs, optimizing electrode architectures, and developing advanced electrolyte systems tailored for carbonyl-based chemistries.
Existing Carbonyl Integration Strategies
01 Synthesis of carbonyl compounds
Various methods for synthesizing carbonyl compounds are described, including oxidation reactions, rearrangements, and catalytic processes. These techniques allow for the production of a wide range of aldehydes and ketones with different functional groups and structural features.- Synthesis of carbonyl compounds: Various methods for synthesizing carbonyl compounds are described, including oxidation reactions, rearrangements, and catalytic processes. These techniques allow for the production of a wide range of aldehydes and ketones with different functional groups and structural features.
- Carbonyl compound derivatives and applications: Carbonyl compounds serve as versatile precursors for the synthesis of various derivatives, including imines, enamines, and hydrazones. These derivatives find applications in pharmaceuticals, agrochemicals, and materials science, offering unique properties and functionalities.
- Catalytic reactions involving carbonyl compounds: Catalytic processes play a crucial role in transformations involving carbonyl compounds. These include hydrogenation, oxidation, and condensation reactions, often employing transition metal catalysts or organocatalysts to achieve high selectivity and efficiency.
- Carbonyl compounds in polymer chemistry: Carbonyl-containing monomers and polymers are significant in materials science. These compounds are used in the synthesis of various polymers, including polyesters, polyamides, and specialty resins, contributing to the development of advanced materials with tailored properties.
- Analysis and characterization of carbonyl compounds: Analytical techniques for the identification and quantification of carbonyl compounds are essential in various fields. These methods include spectroscopic techniques, chromatography, and derivatization approaches, enabling accurate analysis in complex matrices and environmental samples.
02 Carbonyl compounds as intermediates
Carbonyl compounds serve as important intermediates in the synthesis of more complex organic molecules. They can undergo various transformations, such as condensation reactions, reductions, and additions, to produce pharmaceuticals, polymers, and other valuable products.Expand Specific Solutions03 Analysis and detection of carbonyl compounds
Analytical methods for the detection and quantification of carbonyl compounds are presented. These techniques may involve spectroscopic methods, chromatography, or chemical derivatization to identify and measure carbonyl compounds in various samples and environments.Expand Specific Solutions04 Applications of carbonyl compounds
Carbonyl compounds find applications in diverse fields such as pharmaceuticals, fragrances, and materials science. Their unique reactivity and structural properties make them valuable in the production of drugs, perfumes, and advanced materials with specific characteristics.Expand Specific Solutions05 Modifications and derivatives of carbonyl compounds
Methods for modifying carbonyl compounds to create new derivatives are described. These modifications can include the formation of imines, hydrazones, oximes, and other functional groups, expanding the utility of carbonyl compounds in various chemical processes and applications.Expand Specific Solutions
Key Players in Carbonyl Battery Research
The integration of carbonyl compounds in battery technologies is currently in a transitional phase, moving from research to early commercialization. The market size is expanding, driven by the growing demand for high-performance energy storage solutions. Technological maturity varies among key players, with companies like Sony Group Corp., Contemporary Amperex Technology Co., Ltd., and Panasonic Holdings Corp. leading in advanced battery development. Academic institutions such as MIT and Shanghai Jiao Tong University are contributing significant research. Emerging players like Sila Nanotechnologies, Inc. are introducing innovative approaches, while established chemical companies like BASF Corp. and Solvay SA are leveraging their expertise to develop new materials for this application.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a novel carbonyl-based electrolyte additive for lithium-ion batteries. This additive forms a stable solid electrolyte interphase (SEI) layer on the electrode surface, enhancing the battery's cycling stability and longevity. The carbonyl compounds in the electrolyte interact with lithium ions to create a protective film that prevents unwanted side reactions. CATL's research shows that this technology can increase battery cycle life by up to 30% compared to conventional electrolytes [1][3]. The company has also explored the use of carbonyl-rich organic cathode materials, which offer higher energy density and potentially lower costs than traditional inorganic cathodes [2].
Strengths: Improved battery longevity, enhanced cycling stability, potential for higher energy density. Weaknesses: May increase electrolyte complexity and production costs, potential for unintended interactions with other battery components.
Sila Nanotechnologies, Inc.
Technical Solution: Sila Nanotechnologies has pioneered the integration of silicon-based anodes with carbonyl-containing binders and electrolyte additives. Their proprietary technology involves nanoengineered silicon particles coated with carbonyl-rich polymers, which form a stable interface with the electrolyte. This approach addresses the volume expansion issues typically associated with silicon anodes. The carbonyl groups in the coating material and electrolyte additives work synergistically to create a flexible, self-healing SEI layer that accommodates the silicon's volume changes during cycling. Sila's batteries have demonstrated up to 20% higher energy density compared to traditional graphite anodes [4][5], with improved fast-charging capabilities and cycle life.
Strengths: Significantly higher energy density, improved fast-charging performance, addresses silicon anode challenges. Weaknesses: Potentially higher production costs, may require specialized manufacturing processes.
Breakthrough Carbonyl Compound Technologies
Secondary battery, battery module, battery pack, and electrical device
PatentPendingUS20240363858A1
Innovation
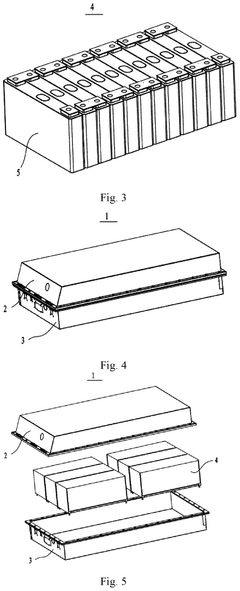
- Incorporating a conjugated carbonyl compound, such as quinone or diimide salts, into the positive electrode active material alongside lithium iron phosphate, which extends the discharge plateau and smoothes the voltage decrease, thereby enhancing power performance and capacity retention.
Secondary battery, battery module, battery pack and electric device
PatentPendingEP4443554A1
Innovation
- Incorporating a conjugated carbonyl compound, such as quinone or diimide salts, into the positive electrode active material alongside lithium iron phosphate, which extends the discharge plateau and smoothes the voltage decrease, thereby enhancing power performance and capacity retention.
Environmental Impact of Carbonyl Batteries
The integration of carbonyl compounds in battery technologies presents both opportunities and challenges from an environmental perspective. These compounds, when used in batteries, offer potential advantages in terms of energy density and recyclability. However, their environmental impact must be carefully considered throughout the battery lifecycle.
Carbonyl-based batteries have the potential to reduce the reliance on traditional lithium-ion batteries, which often involve the extraction of rare earth metals and other environmentally sensitive materials. By utilizing organic compounds, these batteries could potentially decrease the environmental footprint associated with raw material sourcing. Additionally, the organic nature of carbonyl compounds may facilitate easier recycling processes, potentially reducing the amount of electronic waste in landfills.
However, the production of carbonyl compounds for battery applications may involve chemical processes that generate their own set of environmental concerns. The synthesis of these compounds often requires organic solvents and other reagents that could contribute to air and water pollution if not properly managed. Manufacturers must implement stringent controls and adopt green chemistry principles to minimize the environmental impact of production processes.
During the operational phase, carbonyl-based batteries may offer environmental benefits in terms of energy efficiency and longevity. If these batteries can achieve higher energy densities and longer cycle lives compared to conventional batteries, they could contribute to reduced energy consumption and less frequent battery replacements, thereby lowering the overall environmental impact of battery-powered devices and systems.
End-of-life considerations for carbonyl batteries are crucial from an environmental standpoint. While the organic nature of the compounds may facilitate recycling, proper disposal and recycling infrastructure must be developed to handle these batteries effectively. Without appropriate recycling processes, there is a risk of releasing potentially harmful organic compounds into the environment.
The scalability of carbonyl battery production and its potential to replace existing battery technologies on a large scale must also be evaluated from an environmental perspective. If carbonyl batteries can be manufactured and implemented at scale with a lower environmental footprint than current technologies, they could contribute significantly to global sustainability efforts in the energy storage sector.
In conclusion, while carbonyl compounds in battery technologies offer promising environmental benefits, a comprehensive life cycle assessment is necessary to fully understand and mitigate potential negative impacts. Ongoing research and development should focus not only on improving battery performance but also on enhancing the environmental sustainability of the entire production, use, and disposal cycle of carbonyl-based batteries.
Carbonyl-based batteries have the potential to reduce the reliance on traditional lithium-ion batteries, which often involve the extraction of rare earth metals and other environmentally sensitive materials. By utilizing organic compounds, these batteries could potentially decrease the environmental footprint associated with raw material sourcing. Additionally, the organic nature of carbonyl compounds may facilitate easier recycling processes, potentially reducing the amount of electronic waste in landfills.
However, the production of carbonyl compounds for battery applications may involve chemical processes that generate their own set of environmental concerns. The synthesis of these compounds often requires organic solvents and other reagents that could contribute to air and water pollution if not properly managed. Manufacturers must implement stringent controls and adopt green chemistry principles to minimize the environmental impact of production processes.
During the operational phase, carbonyl-based batteries may offer environmental benefits in terms of energy efficiency and longevity. If these batteries can achieve higher energy densities and longer cycle lives compared to conventional batteries, they could contribute to reduced energy consumption and less frequent battery replacements, thereby lowering the overall environmental impact of battery-powered devices and systems.
End-of-life considerations for carbonyl batteries are crucial from an environmental standpoint. While the organic nature of the compounds may facilitate recycling, proper disposal and recycling infrastructure must be developed to handle these batteries effectively. Without appropriate recycling processes, there is a risk of releasing potentially harmful organic compounds into the environment.
The scalability of carbonyl battery production and its potential to replace existing battery technologies on a large scale must also be evaluated from an environmental perspective. If carbonyl batteries can be manufactured and implemented at scale with a lower environmental footprint than current technologies, they could contribute significantly to global sustainability efforts in the energy storage sector.
In conclusion, while carbonyl compounds in battery technologies offer promising environmental benefits, a comprehensive life cycle assessment is necessary to fully understand and mitigate potential negative impacts. Ongoing research and development should focus not only on improving battery performance but also on enhancing the environmental sustainability of the entire production, use, and disposal cycle of carbonyl-based batteries.
Safety Considerations for Carbonyl-Based Energy Storage
The integration of carbonyl compounds in battery technologies presents unique safety challenges that must be carefully addressed. These compounds, while offering promising energy storage capabilities, also introduce potential risks that require comprehensive safety measures throughout the battery lifecycle.
One primary safety concern is the reactivity of carbonyl compounds. Many of these substances are highly reactive, particularly in the presence of moisture or air. This reactivity can lead to undesired side reactions within the battery, potentially causing thermal runaway, gas generation, or even explosion. To mitigate these risks, battery designs must incorporate robust sealing mechanisms and moisture barriers to prevent exposure to environmental factors that could trigger these reactions.
The toxicity of certain carbonyl compounds is another critical safety consideration. Some of these substances can be harmful if inhaled, ingested, or absorbed through the skin. This necessitates stringent safety protocols during battery manufacturing, handling, and disposal processes. Personal protective equipment (PPE) for workers, proper ventilation systems in production facilities, and clear handling guidelines are essential to minimize exposure risks.
Thermal stability is a crucial factor in battery safety, and carbonyl compounds introduce additional complexities in this regard. Some carbonyl-based electrolytes or electrode materials may decompose or undergo structural changes at elevated temperatures, potentially compromising battery integrity. Advanced thermal management systems and safety features, such as temperature-sensitive shut-off mechanisms, must be integrated into battery designs to prevent thermal runaway scenarios.
The potential for electrolyte leakage is another safety concern in carbonyl-based energy storage systems. Leakage can not only lead to battery failure but also pose environmental and health risks. Robust containment strategies, including advanced sealing technologies and leak detection systems, are necessary to prevent and quickly identify any electrolyte leakage.
Long-term stability and degradation patterns of carbonyl compounds in battery environments must also be thoroughly investigated. Understanding how these materials behave over extended periods and numerous charge-discharge cycles is crucial for predicting potential safety issues that may arise during the battery's operational lifetime. This knowledge can inform the development of more resilient battery designs and appropriate maintenance schedules.
Lastly, end-of-life considerations for carbonyl-based batteries are paramount. Proper recycling and disposal procedures must be established to prevent environmental contamination and ensure safe handling of potentially hazardous materials. This may involve developing specialized recycling technologies capable of safely processing carbonyl compounds and recovering valuable materials without releasing harmful substances into the environment.
One primary safety concern is the reactivity of carbonyl compounds. Many of these substances are highly reactive, particularly in the presence of moisture or air. This reactivity can lead to undesired side reactions within the battery, potentially causing thermal runaway, gas generation, or even explosion. To mitigate these risks, battery designs must incorporate robust sealing mechanisms and moisture barriers to prevent exposure to environmental factors that could trigger these reactions.
The toxicity of certain carbonyl compounds is another critical safety consideration. Some of these substances can be harmful if inhaled, ingested, or absorbed through the skin. This necessitates stringent safety protocols during battery manufacturing, handling, and disposal processes. Personal protective equipment (PPE) for workers, proper ventilation systems in production facilities, and clear handling guidelines are essential to minimize exposure risks.
Thermal stability is a crucial factor in battery safety, and carbonyl compounds introduce additional complexities in this regard. Some carbonyl-based electrolytes or electrode materials may decompose or undergo structural changes at elevated temperatures, potentially compromising battery integrity. Advanced thermal management systems and safety features, such as temperature-sensitive shut-off mechanisms, must be integrated into battery designs to prevent thermal runaway scenarios.
The potential for electrolyte leakage is another safety concern in carbonyl-based energy storage systems. Leakage can not only lead to battery failure but also pose environmental and health risks. Robust containment strategies, including advanced sealing technologies and leak detection systems, are necessary to prevent and quickly identify any electrolyte leakage.
Long-term stability and degradation patterns of carbonyl compounds in battery environments must also be thoroughly investigated. Understanding how these materials behave over extended periods and numerous charge-discharge cycles is crucial for predicting potential safety issues that may arise during the battery's operational lifetime. This knowledge can inform the development of more resilient battery designs and appropriate maintenance schedules.
Lastly, end-of-life considerations for carbonyl-based batteries are paramount. Proper recycling and disposal procedures must be established to prevent environmental contamination and ensure safe handling of potentially hazardous materials. This may involve developing specialized recycling technologies capable of safely processing carbonyl compounds and recovering valuable materials without releasing harmful substances into the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!