Revolutionizing Carbonyl Chemistry for Future Innovations
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Chemistry Evolution and Objectives
Carbonyl chemistry has been a cornerstone of organic synthesis for over a century, playing a pivotal role in the development of pharmaceuticals, materials, and industrial processes. The evolution of this field has been marked by significant breakthroughs, from the discovery of the Grignard reaction in the early 1900s to the recent advancements in asymmetric catalysis. As we look towards the future, the objectives for revolutionizing carbonyl chemistry are becoming increasingly ambitious and multifaceted.
The historical trajectory of carbonyl chemistry has been characterized by a continuous expansion of synthetic methodologies. From classical aldol and Claisen condensations to modern transition metal-catalyzed transformations, each era has brought new tools to manipulate these versatile functional groups. The advent of organocatalysis in the early 2000s opened up new avenues for enantioselective carbonyl transformations, significantly impacting the field of asymmetric synthesis.
Current trends in carbonyl chemistry are focused on developing more sustainable and efficient processes. Green chemistry principles are driving research towards atom-economical reactions, catalytic systems that utilize earth-abundant metals, and methodologies that operate under mild conditions. The integration of flow chemistry and continuous processing is also reshaping how carbonyl transformations are conducted on an industrial scale.
Looking ahead, the objectives for revolutionizing carbonyl chemistry are multifold. One primary goal is to achieve perfect chemo-, regio-, and stereoselectivity in carbonyl transformations under benign conditions. This includes the development of catalytic systems capable of distinguishing between subtle electronic and steric differences in complex molecules. Another objective is to harness the power of carbonyl chemistry in the realm of materials science, creating smart materials with tunable properties based on dynamic covalent chemistry.
The intersection of carbonyl chemistry with emerging technologies presents exciting opportunities. Artificial intelligence and machine learning are being employed to predict reaction outcomes and design novel catalysts, potentially accelerating the discovery of unprecedented transformations. Additionally, the application of photochemistry and electrochemistry to carbonyl reactions is opening up new reaction pathways that were previously inaccessible.
A crucial objective in the field is to expand the scope of carbonyl chemistry beyond traditional organic synthesis. This includes exploring the role of carbonyl compounds in biological systems, developing new bioorthogonal reactions for in vivo applications, and creating innovative drug delivery systems based on carbonyl chemistry principles. The potential for carbonyl chemistry to contribute to solving global challenges, such as carbon dioxide utilization and the development of sustainable polymers, is also a key focus area for future innovations.
The historical trajectory of carbonyl chemistry has been characterized by a continuous expansion of synthetic methodologies. From classical aldol and Claisen condensations to modern transition metal-catalyzed transformations, each era has brought new tools to manipulate these versatile functional groups. The advent of organocatalysis in the early 2000s opened up new avenues for enantioselective carbonyl transformations, significantly impacting the field of asymmetric synthesis.
Current trends in carbonyl chemistry are focused on developing more sustainable and efficient processes. Green chemistry principles are driving research towards atom-economical reactions, catalytic systems that utilize earth-abundant metals, and methodologies that operate under mild conditions. The integration of flow chemistry and continuous processing is also reshaping how carbonyl transformations are conducted on an industrial scale.
Looking ahead, the objectives for revolutionizing carbonyl chemistry are multifold. One primary goal is to achieve perfect chemo-, regio-, and stereoselectivity in carbonyl transformations under benign conditions. This includes the development of catalytic systems capable of distinguishing between subtle electronic and steric differences in complex molecules. Another objective is to harness the power of carbonyl chemistry in the realm of materials science, creating smart materials with tunable properties based on dynamic covalent chemistry.
The intersection of carbonyl chemistry with emerging technologies presents exciting opportunities. Artificial intelligence and machine learning are being employed to predict reaction outcomes and design novel catalysts, potentially accelerating the discovery of unprecedented transformations. Additionally, the application of photochemistry and electrochemistry to carbonyl reactions is opening up new reaction pathways that were previously inaccessible.
A crucial objective in the field is to expand the scope of carbonyl chemistry beyond traditional organic synthesis. This includes exploring the role of carbonyl compounds in biological systems, developing new bioorthogonal reactions for in vivo applications, and creating innovative drug delivery systems based on carbonyl chemistry principles. The potential for carbonyl chemistry to contribute to solving global challenges, such as carbon dioxide utilization and the development of sustainable polymers, is also a key focus area for future innovations.
Market Demand for Advanced Carbonyl Compounds
The market demand for advanced carbonyl compounds has been experiencing significant growth, driven by various industries' increasing need for innovative materials and chemical solutions. The pharmaceutical sector stands at the forefront of this demand, as carbonyl compounds play a crucial role in drug discovery and development. With the global pharmaceutical market projected to reach $1.5 trillion by 2023, the demand for novel carbonyl-based drug candidates and intermediates continues to surge.
In the agrochemical industry, advanced carbonyl compounds are essential for developing more effective and environmentally friendly pesticides and herbicides. As the world population grows and arable land becomes scarcer, the need for high-performance agrochemicals is expected to drive the market for specialized carbonyl compounds in this sector.
The polymer and materials science industries are also significant contributors to the demand for advanced carbonyl compounds. These compounds are vital in the development of high-performance plastics, coatings, and adhesives. With the global push towards sustainability, there is a growing interest in bio-based and biodegradable materials, many of which rely on carbonyl chemistry for their synthesis and properties.
The flavor and fragrance industry represents another substantial market for carbonyl compounds. As consumer preferences evolve and the demand for natural and complex flavor profiles increases, the need for sophisticated carbonyl-based aroma compounds continues to grow. This sector is expected to reach $35 billion by 2024, with a significant portion attributed to carbonyl-based ingredients.
In the field of organic electronics, carbonyl compounds are finding new applications in the development of organic light-emitting diodes (OLEDs) and organic photovoltaics. As the demand for more efficient and flexible electronic devices rises, so does the need for advanced carbonyl-based materials that can enhance performance and durability.
The automotive and aerospace industries are also driving demand for advanced carbonyl compounds, particularly in the development of high-performance lubricants, fuel additives, and lightweight composite materials. As these sectors push for greater fuel efficiency and reduced emissions, the role of innovative carbonyl chemistry becomes increasingly important.
Furthermore, the growing focus on green chemistry and sustainable processes is creating new opportunities for carbonyl compounds in catalysis and biocatalysis. Industries are seeking more efficient and environmentally friendly synthetic routes, many of which involve carbonyl chemistry as key steps or intermediates.
In the agrochemical industry, advanced carbonyl compounds are essential for developing more effective and environmentally friendly pesticides and herbicides. As the world population grows and arable land becomes scarcer, the need for high-performance agrochemicals is expected to drive the market for specialized carbonyl compounds in this sector.
The polymer and materials science industries are also significant contributors to the demand for advanced carbonyl compounds. These compounds are vital in the development of high-performance plastics, coatings, and adhesives. With the global push towards sustainability, there is a growing interest in bio-based and biodegradable materials, many of which rely on carbonyl chemistry for their synthesis and properties.
The flavor and fragrance industry represents another substantial market for carbonyl compounds. As consumer preferences evolve and the demand for natural and complex flavor profiles increases, the need for sophisticated carbonyl-based aroma compounds continues to grow. This sector is expected to reach $35 billion by 2024, with a significant portion attributed to carbonyl-based ingredients.
In the field of organic electronics, carbonyl compounds are finding new applications in the development of organic light-emitting diodes (OLEDs) and organic photovoltaics. As the demand for more efficient and flexible electronic devices rises, so does the need for advanced carbonyl-based materials that can enhance performance and durability.
The automotive and aerospace industries are also driving demand for advanced carbonyl compounds, particularly in the development of high-performance lubricants, fuel additives, and lightweight composite materials. As these sectors push for greater fuel efficiency and reduced emissions, the role of innovative carbonyl chemistry becomes increasingly important.
Furthermore, the growing focus on green chemistry and sustainable processes is creating new opportunities for carbonyl compounds in catalysis and biocatalysis. Industries are seeking more efficient and environmentally friendly synthetic routes, many of which involve carbonyl chemistry as key steps or intermediates.
Current Challenges in Carbonyl Synthesis
Carbonyl synthesis remains a cornerstone of organic chemistry, yet it faces several significant challenges that hinder its full potential in modern applications. One of the primary obstacles is the limited substrate scope of many carbonyl-forming reactions. Traditional methods often struggle with sterically hindered or electronically deactivated substrates, limiting the diversity of accessible carbonyl compounds.
The control of stereochemistry in carbonyl synthesis presents another major challenge. While asymmetric catalysis has made significant strides, achieving high enantioselectivity and diastereoselectivity across a broad range of substrates and reaction conditions remains elusive. This is particularly problematic in the synthesis of complex natural products and pharmaceuticals, where precise stereochemical control is crucial.
Sustainability concerns also pose significant challenges in carbonyl chemistry. Many conventional methods rely on toxic reagents, generate substantial waste, or require harsh reaction conditions. The development of greener alternatives that maintain or improve efficiency while reducing environmental impact is an ongoing struggle in the field.
The scalability of carbonyl-forming reactions is another hurdle, especially in industrial applications. Reactions that perform well on a laboratory scale often encounter issues when scaled up, such as decreased yields, longer reaction times, or the formation of unwanted by-products. This challenge is particularly acute for catalytic processes, where catalyst stability and recyclability become critical factors.
Furthermore, the development of novel carbonyl-forming reactions that can access previously challenging or impossible transformations remains an important goal. This includes the direct functionalization of C-H bonds to form carbonyls, the selective oxidation of alcohols under mild conditions, and the development of new C-C bond-forming reactions that generate carbonyl functionalities.
The integration of carbonyl chemistry with emerging technologies presents both opportunities and challenges. For instance, the application of flow chemistry to carbonyl synthesis offers potential benefits in terms of efficiency and scalability, but requires overcoming issues related to reaction kinetics and mixing in continuous flow systems.
Lastly, the predictability and rational design of carbonyl-forming reactions continue to challenge chemists. Despite advances in computational chemistry, accurately modeling complex reaction mechanisms and predicting outcomes, especially for novel transformations, remains difficult. This limitation hampers the development of new methodologies and the optimization of existing processes.
The control of stereochemistry in carbonyl synthesis presents another major challenge. While asymmetric catalysis has made significant strides, achieving high enantioselectivity and diastereoselectivity across a broad range of substrates and reaction conditions remains elusive. This is particularly problematic in the synthesis of complex natural products and pharmaceuticals, where precise stereochemical control is crucial.
Sustainability concerns also pose significant challenges in carbonyl chemistry. Many conventional methods rely on toxic reagents, generate substantial waste, or require harsh reaction conditions. The development of greener alternatives that maintain or improve efficiency while reducing environmental impact is an ongoing struggle in the field.
The scalability of carbonyl-forming reactions is another hurdle, especially in industrial applications. Reactions that perform well on a laboratory scale often encounter issues when scaled up, such as decreased yields, longer reaction times, or the formation of unwanted by-products. This challenge is particularly acute for catalytic processes, where catalyst stability and recyclability become critical factors.
Furthermore, the development of novel carbonyl-forming reactions that can access previously challenging or impossible transformations remains an important goal. This includes the direct functionalization of C-H bonds to form carbonyls, the selective oxidation of alcohols under mild conditions, and the development of new C-C bond-forming reactions that generate carbonyl functionalities.
The integration of carbonyl chemistry with emerging technologies presents both opportunities and challenges. For instance, the application of flow chemistry to carbonyl synthesis offers potential benefits in terms of efficiency and scalability, but requires overcoming issues related to reaction kinetics and mixing in continuous flow systems.
Lastly, the predictability and rational design of carbonyl-forming reactions continue to challenge chemists. Despite advances in computational chemistry, accurately modeling complex reaction mechanisms and predicting outcomes, especially for novel transformations, remains difficult. This limitation hampers the development of new methodologies and the optimization of existing processes.
State-of-the-Art Carbonyl Synthesis Methods
01 Carbonyl compound detection and analysis
Various methods and systems for detecting and analyzing carbonyl compounds in samples. This includes spectroscopic techniques, chemical sensors, and imaging technologies to identify and quantify carbonyl-containing molecules in different matrices.- Carbonyl compound detection and analysis: Various methods and systems for detecting and analyzing carbonyl compounds in samples. These techniques may involve spectroscopic analysis, chemical reactions, or specialized sensors to identify and quantify carbonyl groups in different substances. Such methods are crucial in fields like environmental monitoring, food quality control, and industrial process optimization.
- Carbonyl-based synthesis and reactions: Processes and methods for synthesizing compounds containing carbonyl groups or utilizing carbonyl chemistry in organic reactions. This includes aldol condensations, Grignard reactions, and other transformations involving aldehydes and ketones. These reactions are fundamental in the production of pharmaceuticals, polymers, and fine chemicals.
- Carbonyl compounds in material science: Applications of carbonyl chemistry in material science, including the development of new polymers, coatings, and functional materials. This involves the use of carbonyl-containing monomers or the modification of existing materials through carbonyl reactions to achieve desired properties such as improved durability, adhesion, or reactivity.
- Carbonyl chemistry in biological systems: Study and manipulation of carbonyl compounds in biological contexts, including their role in metabolic processes, protein modifications, and cellular signaling. This area also covers the development of carbonyl-based drugs and the investigation of carbonyl stress in various diseases.
- Carbonyl reduction and oxidation processes: Methods and catalysts for the selective reduction or oxidation of carbonyl compounds. This includes hydrogenation of aldehydes and ketones to alcohols, oxidation of alcohols to carbonyls, and other redox processes involving carbonyl groups. These reactions are important in both industrial and laboratory settings for the synthesis of various organic compounds.
02 Carbonyl-based chemical reactions and synthesis
Processes and methods for synthesizing compounds involving carbonyl groups. This encompasses reactions such as aldol condensations, Grignard reactions, and oxidation of alcohols to aldehydes or ketones, as well as the development of new catalysts and reaction conditions for carbonyl chemistry.Expand Specific Solutions03 Carbonyl compounds in materials science
Applications of carbonyl chemistry in materials science, including the development of polymers, coatings, and advanced materials. This involves using carbonyl-containing monomers or modifying existing materials through carbonyl chemistry to achieve desired properties.Expand Specific Solutions04 Carbonyl chemistry in biological systems
Studies and applications of carbonyl chemistry in biological contexts, such as protein modifications, metabolic processes, and the role of carbonyl compounds in cellular signaling. This also includes the development of carbonyl-based drugs and their interactions with biological targets.Expand Specific Solutions05 Environmental and industrial applications of carbonyl chemistry
Utilization of carbonyl chemistry in environmental monitoring, pollution control, and industrial processes. This includes the development of carbonyl-based sensors for air quality monitoring, methods for removing carbonyl pollutants, and applications in industrial synthesis and manufacturing.Expand Specific Solutions
Key Players in Carbonyl Research and Industry
The field of carbonyl chemistry is experiencing a dynamic competitive landscape, with significant advancements driven by both academic institutions and industry players. The market is in a growth phase, with increasing demand for innovative applications in pharmaceuticals, materials science, and sustainable chemistry. While the exact market size is not specified, the involvement of major chemical companies like BASF, Sumitomo Chemical, and Evonik indicates substantial commercial potential. Technologically, the field is progressing rapidly, with universities such as Zhejiang University, Nagoya University, and Shanghai Jiao Tong University contributing cutting-edge research. Industry leaders like China Petroleum & Chemical Corp. and Nitto Denko are leveraging their R&D capabilities to develop practical applications, suggesting a moderate to high level of technological maturity with room for further innovation.
BASF Corp.
Technical Solution: BASF has developed innovative carbonyl chemistry techniques for sustainable production. They have introduced a novel catalytic process for the synthesis of aldehydes and ketones using CO2 as a carbon source, reducing the carbon footprint of traditional carbonyl compound production[1]. This process employs a proprietary metal-organic framework (MOF) catalyst that enables efficient CO2 activation and incorporation into organic substrates[2]. Additionally, BASF has made strides in photocatalytic carbonyl transformations, utilizing visible light to drive challenging reactions under mild conditions[3]. Their approach combines photoredox catalysis with organocatalysis to achieve selective α-functionalization of aldehydes and ketones[4].
Strengths: Sustainable CO2 utilization, reduced environmental impact, mild reaction conditions. Weaknesses: Potential high costs of catalyst development, scalability challenges for industrial production.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has focused on developing green chemistry approaches to carbonyl transformations. They have pioneered a water-based catalytic system for the selective oxidation of alcohols to aldehydes and ketones, eliminating the need for toxic oxidants[5]. This system utilizes a recyclable ruthenium catalyst supported on carbon nanotubes, achieving high yields and selectivity under mild aqueous conditions[6]. Furthermore, Sumitomo has made significant progress in asymmetric carbonyl chemistry, introducing a series of chiral organocatalysts for enantioselective aldol and Mannich reactions[7]. These catalysts enable the synthesis of complex chiral molecules with high optical purity, crucial for pharmaceutical applications.
Strengths: Environmentally friendly processes, high selectivity, potential for pharmaceutical applications. Weaknesses: Possible limitations in substrate scope, challenges in large-scale implementation of water-based systems.
Breakthrough Innovations in Carbonyl Reactions
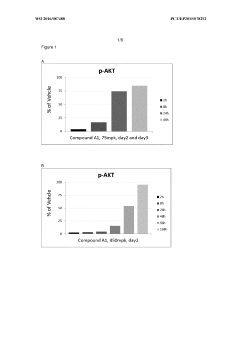
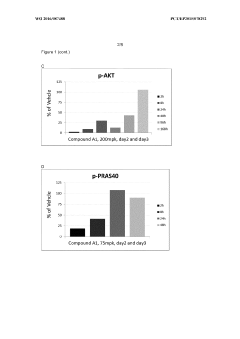
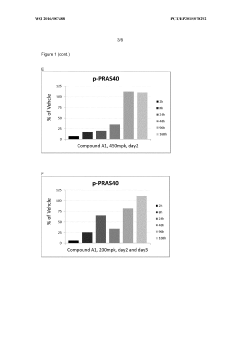
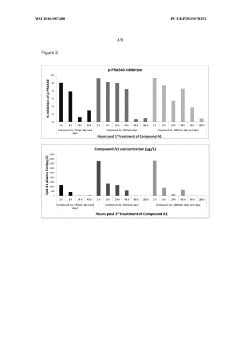
Administration regime for aminoalcohol substituted 2,3-dihydroimidazo[1,2-c]quinazoline derivatives
PatentWO2016087488A1
Innovation
- A novel intermittent dosing regimen for PI3K inhibitors, specifically compounds of formula (I), where the drug is administered for one to five days followed by three to six days of non-administration, providing improved suppression of the PI3K pathway in tumor tissues while reducing side effects.
Method for producing carbonyl compound
PatentInactiveEP1544188A1
Innovation
- A method involving a heterogeneous solution system of an aqueous hydrogen peroxide solution with an oily solution of water-insoluble aliphatic alcohol, using a catalyst from Group 8 to 10 metals, such as platinum or palladium, to produce carbonyl compounds like ketones, aldehydes, or carboxylic acids under mild conditions without the need for additional solvents.
Green Chemistry Approaches in Carbonyl Synthesis
Green chemistry approaches in carbonyl synthesis have gained significant traction in recent years, driven by the need for more sustainable and environmentally friendly chemical processes. These approaches focus on developing methods that minimize waste, reduce energy consumption, and utilize safer reagents and solvents. One key area of innovation is the use of catalytic systems that enable efficient carbonyl formation under mild conditions.
Biocatalysis has emerged as a powerful tool in green carbonyl synthesis. Enzymes such as oxidoreductases and transferases can catalyze carbonyl-forming reactions with high selectivity and efficiency. These biocatalysts often operate in aqueous media, eliminating the need for organic solvents and reducing environmental impact. Recent advances in protein engineering have expanded the scope of enzyme-catalyzed carbonyl synthesis, allowing for the production of complex molecules and pharmaceutical intermediates.
Another promising green approach is the use of renewable feedstocks for carbonyl synthesis. Biomass-derived platform chemicals, such as 5-hydroxymethylfurfural (HMF) and levulinic acid, serve as sustainable precursors for various carbonyl compounds. These bio-based starting materials can be transformed into valuable products through catalytic oxidation, reduction, or condensation reactions, offering alternatives to petroleum-based routes.
Photocatalysis has also gained attention as a green method for carbonyl synthesis. Light-driven reactions, often mediated by visible light photocatalysts, enable the formation of carbonyl compounds under mild conditions and without the need for stoichiometric oxidants. This approach harnesses renewable solar energy and can be coupled with flow chemistry techniques for improved efficiency and scalability.
The development of recyclable and reusable catalysts represents another important aspect of green carbonyl synthesis. Heterogeneous catalysts, such as supported metal nanoparticles or immobilized organocatalysts, can be easily separated from reaction mixtures and reused multiple times. This not only reduces waste generation but also improves the overall economics of the process.
Continuous flow chemistry has emerged as a valuable tool for implementing green carbonyl synthesis at scale. Flow reactors offer improved heat and mass transfer, enabling more efficient and safer reactions. This technology allows for precise control of reaction parameters, facilitating the optimization of green synthetic routes and the integration of multiple steps in a single process.
Biocatalysis has emerged as a powerful tool in green carbonyl synthesis. Enzymes such as oxidoreductases and transferases can catalyze carbonyl-forming reactions with high selectivity and efficiency. These biocatalysts often operate in aqueous media, eliminating the need for organic solvents and reducing environmental impact. Recent advances in protein engineering have expanded the scope of enzyme-catalyzed carbonyl synthesis, allowing for the production of complex molecules and pharmaceutical intermediates.
Another promising green approach is the use of renewable feedstocks for carbonyl synthesis. Biomass-derived platform chemicals, such as 5-hydroxymethylfurfural (HMF) and levulinic acid, serve as sustainable precursors for various carbonyl compounds. These bio-based starting materials can be transformed into valuable products through catalytic oxidation, reduction, or condensation reactions, offering alternatives to petroleum-based routes.
Photocatalysis has also gained attention as a green method for carbonyl synthesis. Light-driven reactions, often mediated by visible light photocatalysts, enable the formation of carbonyl compounds under mild conditions and without the need for stoichiometric oxidants. This approach harnesses renewable solar energy and can be coupled with flow chemistry techniques for improved efficiency and scalability.
The development of recyclable and reusable catalysts represents another important aspect of green carbonyl synthesis. Heterogeneous catalysts, such as supported metal nanoparticles or immobilized organocatalysts, can be easily separated from reaction mixtures and reused multiple times. This not only reduces waste generation but also improves the overall economics of the process.
Continuous flow chemistry has emerged as a valuable tool for implementing green carbonyl synthesis at scale. Flow reactors offer improved heat and mass transfer, enabling more efficient and safer reactions. This technology allows for precise control of reaction parameters, facilitating the optimization of green synthetic routes and the integration of multiple steps in a single process.
Computational Tools for Carbonyl Reaction Design
Computational tools have become indispensable in advancing carbonyl chemistry and driving future innovations. These tools offer powerful capabilities for predicting reaction outcomes, designing novel synthetic routes, and optimizing reaction conditions. Machine learning algorithms, coupled with extensive chemical databases, enable rapid screening of potential carbonyl reactions and identification of promising candidates for further investigation.
Quantum chemical calculations provide detailed insights into reaction mechanisms and transition states, allowing researchers to understand the electronic and structural factors influencing carbonyl reactivity. Density functional theory (DFT) methods, in particular, have proven highly effective in modeling carbonyl compounds and their reactions with various nucleophiles and electrophiles.
Molecular dynamics simulations offer valuable information on the behavior of carbonyl compounds in complex reaction environments, including solvent effects and interactions with catalysts. These simulations can help elucidate the role of non-covalent interactions and dynamic processes in carbonyl chemistry, leading to improved reaction design strategies.
Cheminformatics tools facilitate the analysis of large datasets of carbonyl reactions, enabling the identification of trends and patterns that may not be apparent through traditional experimental approaches. These tools can assist in predicting reaction yields, selectivities, and potential side products, guiding researchers towards more efficient and sustainable synthetic routes.
Virtual screening techniques, combined with structure-based design approaches, are increasingly used to discover novel carbonyl-based compounds with desired properties. This is particularly valuable in drug discovery and materials science, where carbonyl functionalities play crucial roles in molecular recognition and reactivity.
Automated reaction planning systems, incorporating artificial intelligence and expert knowledge, can generate and evaluate multiple synthetic pathways for complex carbonyl-containing target molecules. These systems can significantly accelerate the discovery of new reactions and optimize existing synthetic routes.
As computational power continues to increase and algorithms become more sophisticated, the integration of these tools into experimental workflows is expected to revolutionize carbonyl chemistry. This synergy between computational and experimental approaches will likely lead to unprecedented advances in reaction prediction, catalyst design, and the development of novel carbonyl-based materials and pharmaceuticals.
Quantum chemical calculations provide detailed insights into reaction mechanisms and transition states, allowing researchers to understand the electronic and structural factors influencing carbonyl reactivity. Density functional theory (DFT) methods, in particular, have proven highly effective in modeling carbonyl compounds and their reactions with various nucleophiles and electrophiles.
Molecular dynamics simulations offer valuable information on the behavior of carbonyl compounds in complex reaction environments, including solvent effects and interactions with catalysts. These simulations can help elucidate the role of non-covalent interactions and dynamic processes in carbonyl chemistry, leading to improved reaction design strategies.
Cheminformatics tools facilitate the analysis of large datasets of carbonyl reactions, enabling the identification of trends and patterns that may not be apparent through traditional experimental approaches. These tools can assist in predicting reaction yields, selectivities, and potential side products, guiding researchers towards more efficient and sustainable synthetic routes.
Virtual screening techniques, combined with structure-based design approaches, are increasingly used to discover novel carbonyl-based compounds with desired properties. This is particularly valuable in drug discovery and materials science, where carbonyl functionalities play crucial roles in molecular recognition and reactivity.
Automated reaction planning systems, incorporating artificial intelligence and expert knowledge, can generate and evaluate multiple synthetic pathways for complex carbonyl-containing target molecules. These systems can significantly accelerate the discovery of new reactions and optimize existing synthetic routes.
As computational power continues to increase and algorithms become more sophisticated, the integration of these tools into experimental workflows is expected to revolutionize carbonyl chemistry. This synergy between computational and experimental approaches will likely lead to unprecedented advances in reaction prediction, catalyst design, and the development of novel carbonyl-based materials and pharmaceuticals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!