Carbonyl Compounds: Techniques for Enhanced Catalysis
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Catalysis Background and Objectives
Carbonyl compounds have played a pivotal role in organic synthesis and industrial processes for decades. The catalysis of carbonyl compounds has evolved significantly, driven by the need for more efficient, selective, and environmentally friendly chemical transformations. This field has witnessed remarkable progress, from traditional acid-base catalysis to modern asymmetric and organocatalytic approaches.
The primary objective of enhanced catalysis techniques for carbonyl compounds is to develop more effective and sustainable methods for their transformation. These techniques aim to improve reaction rates, increase product yields, enhance selectivity, and reduce the environmental impact of chemical processes. Researchers and industry professionals are continuously exploring novel catalytic systems that can operate under milder conditions, utilize less toxic reagents, and generate fewer by-products.
One of the key drivers behind the advancement of carbonyl catalysis is the growing demand for optically pure compounds in the pharmaceutical and fine chemical industries. Asymmetric catalysis of carbonyl compounds has emerged as a powerful tool for synthesizing chiral molecules with high enantioselectivity. This has led to the development of various chiral catalysts, including metal complexes, organocatalysts, and enzymes, capable of promoting stereoselective transformations of carbonyl substrates.
Another significant trend in carbonyl catalysis is the shift towards more sustainable and green chemistry practices. This includes the use of renewable feedstocks, the development of recyclable catalysts, and the implementation of atom-economical reactions. Researchers are exploring bio-based catalysts and environmentally benign reaction media to reduce the carbon footprint of carbonyl compound transformations.
The field of carbonyl catalysis is also benefiting from advancements in computational chemistry and high-throughput experimentation. These tools enable researchers to predict reaction outcomes, design novel catalysts, and optimize reaction conditions more efficiently. Machine learning algorithms are increasingly being employed to accelerate the discovery of new catalytic systems and to uncover previously unknown structure-activity relationships.
As we look to the future, the objectives for enhanced catalysis of carbonyl compounds include developing catalysts with broader substrate scope, improving catalyst stability and recyclability, and achieving higher levels of chemo-, regio-, and stereoselectivity. There is also a growing interest in tandem and cascade reactions involving carbonyl compounds, which can lead to more streamlined and efficient synthetic processes.
The primary objective of enhanced catalysis techniques for carbonyl compounds is to develop more effective and sustainable methods for their transformation. These techniques aim to improve reaction rates, increase product yields, enhance selectivity, and reduce the environmental impact of chemical processes. Researchers and industry professionals are continuously exploring novel catalytic systems that can operate under milder conditions, utilize less toxic reagents, and generate fewer by-products.
One of the key drivers behind the advancement of carbonyl catalysis is the growing demand for optically pure compounds in the pharmaceutical and fine chemical industries. Asymmetric catalysis of carbonyl compounds has emerged as a powerful tool for synthesizing chiral molecules with high enantioselectivity. This has led to the development of various chiral catalysts, including metal complexes, organocatalysts, and enzymes, capable of promoting stereoselective transformations of carbonyl substrates.
Another significant trend in carbonyl catalysis is the shift towards more sustainable and green chemistry practices. This includes the use of renewable feedstocks, the development of recyclable catalysts, and the implementation of atom-economical reactions. Researchers are exploring bio-based catalysts and environmentally benign reaction media to reduce the carbon footprint of carbonyl compound transformations.
The field of carbonyl catalysis is also benefiting from advancements in computational chemistry and high-throughput experimentation. These tools enable researchers to predict reaction outcomes, design novel catalysts, and optimize reaction conditions more efficiently. Machine learning algorithms are increasingly being employed to accelerate the discovery of new catalytic systems and to uncover previously unknown structure-activity relationships.
As we look to the future, the objectives for enhanced catalysis of carbonyl compounds include developing catalysts with broader substrate scope, improving catalyst stability and recyclability, and achieving higher levels of chemo-, regio-, and stereoselectivity. There is also a growing interest in tandem and cascade reactions involving carbonyl compounds, which can lead to more streamlined and efficient synthetic processes.
Market Analysis for Carbonyl Compound Applications
The market for carbonyl compound applications has experienced significant growth in recent years, driven by increasing demand across various industries. Carbonyl compounds, characterized by their C=O functional group, play a crucial role in numerous chemical processes and products. The global market for carbonyl compounds is projected to expand at a steady rate, with key applications in pharmaceuticals, agrochemicals, and fine chemicals sectors.
In the pharmaceutical industry, carbonyl compounds serve as essential building blocks for drug synthesis. The growing prevalence of chronic diseases and the continuous development of new therapeutic agents have fueled the demand for carbonyl compounds in this sector. Aldol reactions, Mannich reactions, and other carbonyl-based transformations are widely employed in the production of active pharmaceutical ingredients (APIs) and intermediates.
The agrochemical sector represents another significant market for carbonyl compounds. These compounds are utilized in the synthesis of pesticides, herbicides, and fungicides, which are crucial for enhancing crop yields and protecting agricultural products. As global food demand continues to rise, the need for effective agrochemicals is expected to drive the market for carbonyl compounds in this sector.
The fine chemicals industry also relies heavily on carbonyl compounds for the production of fragrances, flavors, and specialty chemicals. The growing consumer preference for natural and organic products has led to increased demand for carbonyl-based compounds derived from renewable sources. This trend has opened up new opportunities for bio-based carbonyl compounds and green chemistry approaches in the market.
Geographically, North America and Europe have traditionally been the largest markets for carbonyl compound applications, owing to their well-established pharmaceutical and chemical industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization, rising disposable incomes, and growing investments in research and development.
The market for carbonyl compounds is characterized by intense competition among key players, including major chemical companies and specialty chemical manufacturers. These companies are focusing on developing innovative catalytic processes to enhance the efficiency and selectivity of carbonyl compound transformations. The push for sustainable and environmentally friendly production methods has also led to increased research in bio-catalysis and green chemistry approaches for carbonyl compound synthesis.
In conclusion, the market for carbonyl compound applications is poised for continued growth, driven by diverse industrial applications and ongoing technological advancements. The development of novel catalytic techniques for enhanced carbonyl compound transformations is expected to play a crucial role in shaping the future of this market, offering opportunities for improved efficiency, selectivity, and sustainability across various sectors.
In the pharmaceutical industry, carbonyl compounds serve as essential building blocks for drug synthesis. The growing prevalence of chronic diseases and the continuous development of new therapeutic agents have fueled the demand for carbonyl compounds in this sector. Aldol reactions, Mannich reactions, and other carbonyl-based transformations are widely employed in the production of active pharmaceutical ingredients (APIs) and intermediates.
The agrochemical sector represents another significant market for carbonyl compounds. These compounds are utilized in the synthesis of pesticides, herbicides, and fungicides, which are crucial for enhancing crop yields and protecting agricultural products. As global food demand continues to rise, the need for effective agrochemicals is expected to drive the market for carbonyl compounds in this sector.
The fine chemicals industry also relies heavily on carbonyl compounds for the production of fragrances, flavors, and specialty chemicals. The growing consumer preference for natural and organic products has led to increased demand for carbonyl-based compounds derived from renewable sources. This trend has opened up new opportunities for bio-based carbonyl compounds and green chemistry approaches in the market.
Geographically, North America and Europe have traditionally been the largest markets for carbonyl compound applications, owing to their well-established pharmaceutical and chemical industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization, rising disposable incomes, and growing investments in research and development.
The market for carbonyl compounds is characterized by intense competition among key players, including major chemical companies and specialty chemical manufacturers. These companies are focusing on developing innovative catalytic processes to enhance the efficiency and selectivity of carbonyl compound transformations. The push for sustainable and environmentally friendly production methods has also led to increased research in bio-catalysis and green chemistry approaches for carbonyl compound synthesis.
In conclusion, the market for carbonyl compound applications is poised for continued growth, driven by diverse industrial applications and ongoing technological advancements. The development of novel catalytic techniques for enhanced carbonyl compound transformations is expected to play a crucial role in shaping the future of this market, offering opportunities for improved efficiency, selectivity, and sustainability across various sectors.
Current Challenges in Carbonyl Catalysis
Carbonyl catalysis faces several significant challenges that hinder its widespread application and efficiency. One of the primary obstacles is catalyst selectivity. Many carbonyl compounds possess multiple reactive sites, making it difficult to achieve precise control over the desired reaction pathway. This often leads to unwanted side reactions and reduced product yields.
Another major challenge is catalyst stability under various reaction conditions. Carbonyl catalysts are often sensitive to moisture, air, and temperature fluctuations, which can lead to deactivation or degradation of the catalyst. This sensitivity limits the scope of reactions that can be performed and necessitates stringent control over reaction environments, increasing operational costs and complexity.
The issue of catalyst recyclability and reusability also presents a significant hurdle. Many carbonyl catalysts are homogeneous in nature, making their separation and recovery from reaction mixtures challenging. This not only increases the overall cost of the process but also raises environmental concerns due to potential metal contamination in the final products.
Catalyst loading is another area of concern. Current carbonyl catalysis often requires relatively high catalyst concentrations to achieve satisfactory reaction rates and yields. This impacts the economic viability of large-scale applications and can introduce additional purification steps to remove residual catalyst from the product.
The development of sustainable and green catalytic processes remains a challenge in carbonyl catalysis. Many traditional catalysts rely on precious metals or environmentally harmful substances. There is a growing need for catalysts based on earth-abundant elements and environmentally benign materials that can match or exceed the performance of conventional systems.
Substrate scope limitation is also a significant issue. Many carbonyl catalysts exhibit high efficiency for specific substrates but fail to maintain their activity across a broader range of carbonyl compounds. This narrow substrate scope restricts the versatility of catalytic systems and their potential applications in diverse synthetic processes.
Finally, the challenge of scaling up laboratory-scale reactions to industrial processes persists. Factors such as heat and mass transfer limitations, catalyst deactivation rates, and process economics often become more pronounced at larger scales, necessitating significant optimization and sometimes complete redesign of catalytic systems.
Another major challenge is catalyst stability under various reaction conditions. Carbonyl catalysts are often sensitive to moisture, air, and temperature fluctuations, which can lead to deactivation or degradation of the catalyst. This sensitivity limits the scope of reactions that can be performed and necessitates stringent control over reaction environments, increasing operational costs and complexity.
The issue of catalyst recyclability and reusability also presents a significant hurdle. Many carbonyl catalysts are homogeneous in nature, making their separation and recovery from reaction mixtures challenging. This not only increases the overall cost of the process but also raises environmental concerns due to potential metal contamination in the final products.
Catalyst loading is another area of concern. Current carbonyl catalysis often requires relatively high catalyst concentrations to achieve satisfactory reaction rates and yields. This impacts the economic viability of large-scale applications and can introduce additional purification steps to remove residual catalyst from the product.
The development of sustainable and green catalytic processes remains a challenge in carbonyl catalysis. Many traditional catalysts rely on precious metals or environmentally harmful substances. There is a growing need for catalysts based on earth-abundant elements and environmentally benign materials that can match or exceed the performance of conventional systems.
Substrate scope limitation is also a significant issue. Many carbonyl catalysts exhibit high efficiency for specific substrates but fail to maintain their activity across a broader range of carbonyl compounds. This narrow substrate scope restricts the versatility of catalytic systems and their potential applications in diverse synthetic processes.
Finally, the challenge of scaling up laboratory-scale reactions to industrial processes persists. Factors such as heat and mass transfer limitations, catalyst deactivation rates, and process economics often become more pronounced at larger scales, necessitating significant optimization and sometimes complete redesign of catalytic systems.
Existing Enhanced Catalysis Methods
01 Catalytic oxidation of carbonyl compounds
Various catalytic systems are employed for the oxidation of carbonyl compounds. These processes involve the use of metal-based catalysts or organocatalysts to facilitate the conversion of aldehydes or ketones into higher oxidation state products such as carboxylic acids or esters. The catalysts can be homogeneous or heterogeneous, and the reactions often occur under mild conditions.- Catalytic oxidation of carbonyl compounds: Various catalytic systems are employed for the oxidation of carbonyl compounds. These processes involve the use of metal-based catalysts or enzymes to facilitate the conversion of aldehydes and ketones into higher oxidation state products such as carboxylic acids or esters. The catalysts can be homogeneous or heterogeneous, and the reactions often occur under mild conditions to improve selectivity and yield.
- Catalytic reduction of carbonyl compounds: Catalytic reduction of carbonyl compounds is an important process in organic synthesis. This involves the use of transition metal catalysts, often in combination with hydrogen gas or hydride sources, to convert aldehydes and ketones into alcohols. The choice of catalyst and reaction conditions can influence the stereoselectivity and efficiency of the reduction process.
- Catalytic C-C bond formation involving carbonyl compounds: Catalysts play a crucial role in facilitating C-C bond formation reactions involving carbonyl compounds. These include aldol condensations, Knoevenagel condensations, and various cross-coupling reactions. The catalysts can be Lewis acids, organocatalysts, or transition metal complexes, enabling the formation of new carbon-carbon bonds under mild conditions with high selectivity.
- Biocatalysis of carbonyl compounds: Enzymes are used as biocatalysts for various transformations of carbonyl compounds. These include reductions, oxidations, and C-C bond formations. Biocatalytic processes often offer advantages such as high selectivity, mild reaction conditions, and environmental friendliness. Enzyme engineering and immobilization techniques are employed to enhance the efficiency and reusability of these biocatalysts.
- Catalytic functionalization of carbonyl compounds: Various catalytic systems are developed for the functionalization of carbonyl compounds. These processes involve the addition of different functional groups to the carbonyl moiety or adjacent positions. Examples include catalytic hydrogenation, hydroformylation, and various C-H activation reactions. The catalysts used can be homogeneous or heterogeneous, and often involve transition metal complexes or organocatalysts.
02 Catalytic reduction of carbonyl compounds
Catalytic reduction of carbonyl compounds is an important process in organic synthesis. This involves the use of transition metal catalysts, often in combination with hydrogen gas or hydride sources, to convert aldehydes and ketones into alcohols. The choice of catalyst and reaction conditions can influence the selectivity and efficiency of the reduction process.Expand Specific Solutions03 Asymmetric catalysis in carbonyl transformations
Asymmetric catalysis plays a crucial role in the synthesis of chiral compounds from carbonyl precursors. This involves the use of chiral catalysts to selectively form one enantiomer or diastereomer over the other. Applications include asymmetric hydrogenation, aldol reactions, and Michael additions, which are important in the pharmaceutical and fine chemical industries.Expand Specific Solutions04 Catalytic C-C bond formation involving carbonyl compounds
Catalytic methods for carbon-carbon bond formation using carbonyl compounds as substrates or reagents are widely studied. These include aldol condensations, Knoevenagel condensations, and various cross-coupling reactions. The catalysts used can be Lewis acids, bases, or transition metal complexes, enabling the formation of complex molecular structures from simple carbonyl starting materials.Expand Specific Solutions05 Enzymatic catalysis of carbonyl compounds
Enzymes serve as highly selective and efficient catalysts for various transformations of carbonyl compounds in biological systems and industrial applications. These biocatalysts can perform oxidations, reductions, and C-C bond formations under mild conditions with high stereoselectivity. The use of engineered enzymes and whole-cell biocatalysts has expanded the scope of these transformations in green chemistry applications.Expand Specific Solutions
Key Players in Carbonyl Catalysis Research
The field of carbonyl compound catalysis is in a mature stage of development, with ongoing research focused on enhancing efficiency and selectivity. The market size is substantial, driven by applications in pharmaceuticals, fine chemicals, and materials science. Technologically, the field is well-established but continues to evolve. Companies like BASF, Sumitomo Chemical, and China Petroleum & Chemical Corp. are major players, leveraging their extensive R&D capabilities. Smaller, specialized firms such as Novomer and Econic Technologies are pushing boundaries in CO2 utilization. Academic institutions like Cornell University and Northwestern University contribute significantly to fundamental research, while national laboratories like Advanced Industrial Science & Technology provide crucial support for innovation in this field.
BASF Corp.
Technical Solution: BASF has developed innovative catalytic systems for carbonyl compounds, focusing on asymmetric hydrogenation. Their approach utilizes chiral transition metal complexes, particularly ruthenium-based catalysts, to achieve high enantioselectivity in the reduction of ketones and aldehydes[1]. The company has also made significant progress in oxidation catalysis, employing metal-organic frameworks (MOFs) as heterogeneous catalysts for the selective oxidation of alcohols to carbonyl compounds[3]. BASF's research extends to photocatalytic methods, using visible light-activated catalysts to promote carbonyl formation under mild conditions, reducing energy consumption and improving sustainability[5].
Strengths: Wide range of catalytic solutions, high enantioselectivity, and sustainable approaches. Weaknesses: Potential high costs of precious metal catalysts and complexity in large-scale implementation.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has focused on developing organocatalysts for carbonyl compound transformations. Their approach centers on proline-derived catalysts for aldol reactions and Michael additions, achieving high yields and stereoselectivity[2]. The company has also made strides in cross-coupling reactions involving carbonyl compounds, utilizing palladium-based catalysts with tailored ligands to enhance reactivity and selectivity[4]. Additionally, Sumitomo has explored enzymatic catalysis for carbonyl reductions, employing engineered ketoreductases to produce chiral alcohols with excellent optical purity[6].
Strengths: Diverse catalytic portfolio, high stereoselectivity, and eco-friendly enzymatic approaches. Weaknesses: Potential scalability issues with some organocatalysts and enzyme stability concerns.
Innovative Approaches in Carbonyl Catalysis
Catalyst for alkylation of carbonyl compounds, process for preparation and application thereof
PatentActiveIN201811036001A
Innovation
- A catalyst of Formula (I), specifically a NNN-Ni complex, is developed for the alkylation of carbonyl compounds, utilizing a process that involves the preparation of 2,6-bis(morpholinomethyl)pyridine and subsequent reaction with hydrated nickel salts, allowing for the activation of O-H and C-H bonds using alcohols as alkylating agents under mild conditions.
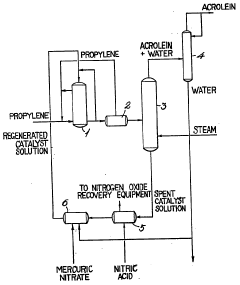
Method of preparing carbonyl compounds from olefinic hydrocarbons
PatentInactiveGB1012336A
Innovation
- The method involves using mercuric nitrate as an oxidizing agent in an aqueous suspension or solution, with temperatures ranging from 0 to 180°C, allowing for the formation of carbonyl compounds while facilitating easy regeneration of the oxidizing agent, and optionally incorporating other metal nitrates to adjust product yields and compositions.
Environmental Impact of Carbonyl Catalysis
The environmental impact of carbonyl catalysis is a critical consideration in the development and application of enhanced catalytic techniques for carbonyl compounds. These processes, while offering significant benefits in terms of efficiency and selectivity, also present potential environmental challenges that must be carefully addressed.
One of the primary environmental concerns associated with carbonyl catalysis is the use of metal-based catalysts. Many traditional catalysts for carbonyl transformations contain heavy metals such as palladium, platinum, or rhodium. These metals, while highly effective, can pose risks to ecosystems if released into the environment. Leaching of metal catalysts into waste streams can lead to soil and water contamination, potentially affecting aquatic life and entering the food chain.
To mitigate these risks, researchers are increasingly focusing on the development of more environmentally benign catalysts. This includes the exploration of earth-abundant metals like iron and nickel as alternatives to precious metals. Additionally, there is growing interest in metal-free organocatalysts, which can significantly reduce the environmental footprint of carbonyl catalysis processes.
The solvents used in carbonyl catalysis also contribute to its environmental impact. Many traditional organic solvents are volatile organic compounds (VOCs) that can contribute to air pollution and ozone depletion. In response, there is a shift towards using greener solvents such as water, supercritical CO2, or ionic liquids. These alternatives not only reduce environmental harm but often enhance catalyst performance and product selectivity.
Energy consumption is another crucial factor in assessing the environmental impact of carbonyl catalysis. While catalysts generally reduce the energy requirements of chemical reactions, the overall process can still be energy-intensive, particularly in industrial settings. Efforts to improve energy efficiency include the development of photocatalytic and electrocatalytic systems that can operate under milder conditions and utilize renewable energy sources.
Waste generation and management are also significant environmental considerations. Carbonyl catalysis often involves the use of stoichiometric reagents and produces byproducts that require disposal. Advances in atom economy and the design of more selective catalysts are helping to minimize waste production. Furthermore, the implementation of continuous flow processes and microreactor technologies is enabling more efficient use of resources and easier separation of products and catalysts.
The life cycle assessment (LCA) of carbonyl catalysis processes is becoming increasingly important in evaluating their overall environmental impact. This holistic approach considers factors such as raw material extraction, catalyst synthesis, reaction conditions, and product purification. LCA studies are guiding the development of more sustainable catalytic systems and informing decision-making in industrial applications.
One of the primary environmental concerns associated with carbonyl catalysis is the use of metal-based catalysts. Many traditional catalysts for carbonyl transformations contain heavy metals such as palladium, platinum, or rhodium. These metals, while highly effective, can pose risks to ecosystems if released into the environment. Leaching of metal catalysts into waste streams can lead to soil and water contamination, potentially affecting aquatic life and entering the food chain.
To mitigate these risks, researchers are increasingly focusing on the development of more environmentally benign catalysts. This includes the exploration of earth-abundant metals like iron and nickel as alternatives to precious metals. Additionally, there is growing interest in metal-free organocatalysts, which can significantly reduce the environmental footprint of carbonyl catalysis processes.
The solvents used in carbonyl catalysis also contribute to its environmental impact. Many traditional organic solvents are volatile organic compounds (VOCs) that can contribute to air pollution and ozone depletion. In response, there is a shift towards using greener solvents such as water, supercritical CO2, or ionic liquids. These alternatives not only reduce environmental harm but often enhance catalyst performance and product selectivity.
Energy consumption is another crucial factor in assessing the environmental impact of carbonyl catalysis. While catalysts generally reduce the energy requirements of chemical reactions, the overall process can still be energy-intensive, particularly in industrial settings. Efforts to improve energy efficiency include the development of photocatalytic and electrocatalytic systems that can operate under milder conditions and utilize renewable energy sources.
Waste generation and management are also significant environmental considerations. Carbonyl catalysis often involves the use of stoichiometric reagents and produces byproducts that require disposal. Advances in atom economy and the design of more selective catalysts are helping to minimize waste production. Furthermore, the implementation of continuous flow processes and microreactor technologies is enabling more efficient use of resources and easier separation of products and catalysts.
The life cycle assessment (LCA) of carbonyl catalysis processes is becoming increasingly important in evaluating their overall environmental impact. This holistic approach considers factors such as raw material extraction, catalyst synthesis, reaction conditions, and product purification. LCA studies are guiding the development of more sustainable catalytic systems and informing decision-making in industrial applications.
Economic Viability of Advanced Catalytic Methods
The economic viability of advanced catalytic methods for carbonyl compounds is a critical consideration in the development and implementation of enhanced catalysis techniques. These methods have the potential to significantly impact industrial processes, particularly in the pharmaceutical and fine chemical sectors. The cost-effectiveness of these advanced techniques is largely dependent on several factors, including catalyst efficiency, selectivity, and recyclability.
One of the primary economic drivers for advanced catalytic methods is the potential for increased yield and reduced waste generation. By improving selectivity and conversion rates, these techniques can lead to more efficient use of raw materials and energy resources. This not only reduces production costs but also aligns with sustainability goals, which are increasingly important in today's regulatory environment.
The initial investment in advanced catalytic systems can be substantial, often requiring specialized equipment and highly purified reagents. However, the long-term economic benefits can outweigh these upfront costs. For instance, the use of heterogeneous catalysts that can be easily separated and reused multiple times can significantly reduce operational expenses over time. Additionally, the development of more robust catalysts with extended lifetimes can decrease the frequency of catalyst replacement, further improving the economic outlook.
Another aspect to consider is the potential for process intensification. Advanced catalytic methods often allow for reactions to be carried out under milder conditions or in fewer steps. This can lead to reduced energy consumption and smaller plant footprints, translating to lower capital and operational expenditures. Furthermore, the ability to conduct reactions in continuous flow systems, rather than batch processes, can enhance productivity and reduce labor costs.
The economic viability of these methods is also influenced by market demands for high-purity products. In the pharmaceutical industry, where stringent quality standards are paramount, advanced catalytic techniques that offer improved product purity can command premium prices. This can offset the higher costs associated with implementing these sophisticated catalytic systems.
However, it is important to note that the economic feasibility of advanced catalytic methods can vary significantly depending on the specific application and scale of production. Small-scale, high-value product manufacturing may justify more expensive catalytic systems, while large-scale commodity chemical production may require more cost-competitive solutions. As such, a thorough economic analysis, considering factors such as raw material costs, energy prices, and market demand, is essential for determining the viability of implementing advanced catalytic methods in any given scenario.
One of the primary economic drivers for advanced catalytic methods is the potential for increased yield and reduced waste generation. By improving selectivity and conversion rates, these techniques can lead to more efficient use of raw materials and energy resources. This not only reduces production costs but also aligns with sustainability goals, which are increasingly important in today's regulatory environment.
The initial investment in advanced catalytic systems can be substantial, often requiring specialized equipment and highly purified reagents. However, the long-term economic benefits can outweigh these upfront costs. For instance, the use of heterogeneous catalysts that can be easily separated and reused multiple times can significantly reduce operational expenses over time. Additionally, the development of more robust catalysts with extended lifetimes can decrease the frequency of catalyst replacement, further improving the economic outlook.
Another aspect to consider is the potential for process intensification. Advanced catalytic methods often allow for reactions to be carried out under milder conditions or in fewer steps. This can lead to reduced energy consumption and smaller plant footprints, translating to lower capital and operational expenditures. Furthermore, the ability to conduct reactions in continuous flow systems, rather than batch processes, can enhance productivity and reduce labor costs.
The economic viability of these methods is also influenced by market demands for high-purity products. In the pharmaceutical industry, where stringent quality standards are paramount, advanced catalytic techniques that offer improved product purity can command premium prices. This can offset the higher costs associated with implementing these sophisticated catalytic systems.
However, it is important to note that the economic feasibility of advanced catalytic methods can vary significantly depending on the specific application and scale of production. Small-scale, high-value product manufacturing may justify more expensive catalytic systems, while large-scale commodity chemical production may require more cost-competitive solutions. As such, a thorough economic analysis, considering factors such as raw material costs, energy prices, and market demand, is essential for determining the viability of implementing advanced catalytic methods in any given scenario.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!