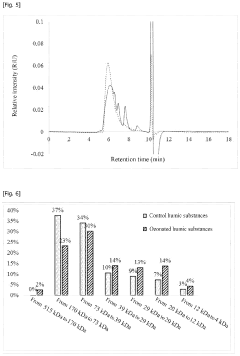
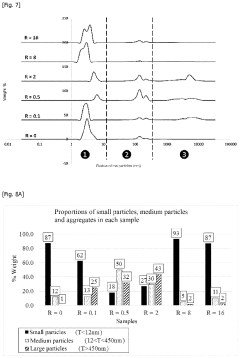
Compare Physicochemical Properties of Fulvic Acid Sources
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fulvic Acid Background and Research Objectives
Fulvic acid represents a complex mixture of heterogeneous organic compounds derived from the decomposition of plant and animal materials. This natural substance has gained significant attention in recent decades due to its remarkable physicochemical properties and potential applications across multiple industries. Historically, fulvic acid research began in the early 20th century as part of broader investigations into soil organic matter, but dedicated research specifically on fulvic acid properties accelerated in the 1960s with improved analytical techniques.
The evolution of fulvic acid technology has progressed from basic extraction methods to sophisticated fractionation and characterization approaches. Initially viewed primarily as a soil amendment, fulvic acid applications have expanded dramatically to include human and animal nutrition, pharmaceutical formulations, environmental remediation, and industrial processes. This expansion reflects growing recognition of its unique molecular structure and functional capabilities.
Current technological trends indicate increasing focus on standardization of fulvic acid characterization methods, as the diverse sources of this compound result in significant variability in composition and properties. The scientific community is moving toward more precise analytical frameworks to enable meaningful comparisons between fulvic acids derived from different sources such as soil, peat, leonardite, and aquatic environments.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of the physicochemical properties of fulvic acids obtained from diverse geological and biological sources. Specifically, we aim to establish quantitative relationships between source materials and resulting fulvic acid characteristics including molecular weight distribution, functional group composition, metal-binding capacity, redox properties, and bioactivity profiles.
Secondary objectives include developing standardized analytical protocols for fulvic acid characterization that can be universally applied across different source materials, identifying optimal extraction and purification methodologies for specific applications, and correlating specific physicochemical properties with functional performance in various application contexts.
This research addresses a critical knowledge gap in the field, as current literature lacks systematic comparative studies that control for extraction methodology while examining source-dependent variations. By establishing clear relationships between fulvic acid sources and their resulting properties, this work will enable more targeted development of fulvic acid products for specific applications and facilitate quality control measures for commercial products.
The anticipated technological outcomes include a comprehensive database of fulvic acid properties indexed by source material, predictive models for estimating functional performance based on measurable physicochemical parameters, and optimized extraction protocols tailored to specific source materials and intended applications.
The evolution of fulvic acid technology has progressed from basic extraction methods to sophisticated fractionation and characterization approaches. Initially viewed primarily as a soil amendment, fulvic acid applications have expanded dramatically to include human and animal nutrition, pharmaceutical formulations, environmental remediation, and industrial processes. This expansion reflects growing recognition of its unique molecular structure and functional capabilities.
Current technological trends indicate increasing focus on standardization of fulvic acid characterization methods, as the diverse sources of this compound result in significant variability in composition and properties. The scientific community is moving toward more precise analytical frameworks to enable meaningful comparisons between fulvic acids derived from different sources such as soil, peat, leonardite, and aquatic environments.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of the physicochemical properties of fulvic acids obtained from diverse geological and biological sources. Specifically, we aim to establish quantitative relationships between source materials and resulting fulvic acid characteristics including molecular weight distribution, functional group composition, metal-binding capacity, redox properties, and bioactivity profiles.
Secondary objectives include developing standardized analytical protocols for fulvic acid characterization that can be universally applied across different source materials, identifying optimal extraction and purification methodologies for specific applications, and correlating specific physicochemical properties with functional performance in various application contexts.
This research addresses a critical knowledge gap in the field, as current literature lacks systematic comparative studies that control for extraction methodology while examining source-dependent variations. By establishing clear relationships between fulvic acid sources and their resulting properties, this work will enable more targeted development of fulvic acid products for specific applications and facilitate quality control measures for commercial products.
The anticipated technological outcomes include a comprehensive database of fulvic acid properties indexed by source material, predictive models for estimating functional performance based on measurable physicochemical parameters, and optimized extraction protocols tailored to specific source materials and intended applications.
Market Analysis of Fulvic Acid Products
The global fulvic acid market has experienced significant growth in recent years, with an estimated market value reaching $1.5 billion in 2023. This growth trajectory is expected to continue at a compound annual growth rate of approximately 6.8% through 2030, driven primarily by increasing demand in agriculture, pharmaceutical, and cosmetic industries. The agricultural sector currently dominates market consumption, accounting for nearly 65% of total fulvic acid usage worldwide.
Regional analysis reveals that North America and Europe hold the largest market shares, collectively representing about 58% of the global market. However, the Asia-Pacific region, particularly China and India, is emerging as the fastest-growing market due to expanding agricultural activities and increasing awareness about soil health. Latin America, especially Brazil, has also shown substantial growth potential in recent years.
The market segmentation of fulvic acid products reveals diverse applications across industries. In agriculture, fulvic acid is primarily marketed as a soil conditioner and plant growth enhancer, with liquid formulations dominating this segment. The pharmaceutical and nutraceutical industries utilize fulvic acid in dietary supplements, representing approximately 20% of the market. Cosmetic applications, though smaller at about 10% of the market, show the highest growth rate at nearly 8.5% annually.
Consumer trends indicate a growing preference for organic and natural products, which has significantly benefited the fulvic acid market. The increasing adoption of sustainable farming practices and organic food production has created substantial demand for natural soil amendments and plant growth stimulants. Additionally, the wellness industry's focus on natural supplements has boosted fulvic acid's presence in health products.
Price analysis shows considerable variation based on source and purity levels. Premium fulvic acid extracted from high-quality leonardite or humic shale commands prices between $15-25 per kilogram for industrial grade and $50-80 per kilogram for pharmaceutical grade. Products derived from composted materials typically sell at lower price points, ranging from $8-15 per kilogram, reflecting their generally lower concentration and purity levels.
Distribution channels have evolved significantly, with e-commerce platforms gaining substantial market share, now accounting for approximately 30% of retail sales. Traditional agricultural supply chains remain important for bulk purchases, while specialty health stores serve as key distribution points for supplement products. Direct-to-consumer models have also emerged, particularly for premium and specialized fulvic acid formulations.
Regional analysis reveals that North America and Europe hold the largest market shares, collectively representing about 58% of the global market. However, the Asia-Pacific region, particularly China and India, is emerging as the fastest-growing market due to expanding agricultural activities and increasing awareness about soil health. Latin America, especially Brazil, has also shown substantial growth potential in recent years.
The market segmentation of fulvic acid products reveals diverse applications across industries. In agriculture, fulvic acid is primarily marketed as a soil conditioner and plant growth enhancer, with liquid formulations dominating this segment. The pharmaceutical and nutraceutical industries utilize fulvic acid in dietary supplements, representing approximately 20% of the market. Cosmetic applications, though smaller at about 10% of the market, show the highest growth rate at nearly 8.5% annually.
Consumer trends indicate a growing preference for organic and natural products, which has significantly benefited the fulvic acid market. The increasing adoption of sustainable farming practices and organic food production has created substantial demand for natural soil amendments and plant growth stimulants. Additionally, the wellness industry's focus on natural supplements has boosted fulvic acid's presence in health products.
Price analysis shows considerable variation based on source and purity levels. Premium fulvic acid extracted from high-quality leonardite or humic shale commands prices between $15-25 per kilogram for industrial grade and $50-80 per kilogram for pharmaceutical grade. Products derived from composted materials typically sell at lower price points, ranging from $8-15 per kilogram, reflecting their generally lower concentration and purity levels.
Distribution channels have evolved significantly, with e-commerce platforms gaining substantial market share, now accounting for approximately 30% of retail sales. Traditional agricultural supply chains remain important for bulk purchases, while specialty health stores serve as key distribution points for supplement products. Direct-to-consumer models have also emerged, particularly for premium and specialized fulvic acid formulations.
Current Challenges in Fulvic Acid Characterization
Despite significant advancements in analytical techniques, the characterization of fulvic acids (FAs) remains fraught with challenges that impede comprehensive comparison across different sources. The inherent heterogeneity of fulvic acids presents a fundamental obstacle, as these complex mixtures contain thousands of individual compounds with varying molecular weights, functional groups, and structural configurations. This molecular diversity makes standardized analysis exceptionally difficult, often resulting in inconsistent characterization methodologies across research groups.
Extraction and isolation procedures introduce another layer of complexity, as different protocols can selectively isolate specific FA fractions, potentially altering the native properties of the original material. The lack of universally accepted extraction standards means that comparisons between studies often involve samples prepared under divergent conditions, raising questions about data comparability and reproducibility.
Analytical limitations further compound these challenges. Traditional techniques such as elemental analysis provide only bulk properties, while more advanced methods like nuclear magnetic resonance (NMR) spectroscopy often yield complex spectra that are difficult to interpret definitively. High-resolution mass spectrometry, while powerful, captures only ionizable components and may miss significant portions of the FA composition.
Environmental factors introduce additional variables, as the physicochemical properties of FAs can be significantly influenced by pH, ionic strength, temperature, and the presence of metal ions. These contextual dependencies mean that properties measured under laboratory conditions may not accurately reflect behavior in natural or application environments.
The absence of reference standards represents perhaps the most critical challenge. Unlike pure compounds, no universal fulvic acid standard exists against which samples can be calibrated. This fundamental limitation undermines efforts to establish quantitative relationships between structural features and functional properties across different FA sources.
Interdisciplinary barriers further complicate the field, as researchers from soil science, aquatic chemistry, and commercial applications often employ different terminologies and methodological approaches. This fragmentation of knowledge impedes the development of comprehensive characterization frameworks that could facilitate meaningful comparisons.
Recent technological advances in multi-dimensional chromatography, advanced spectroscopic techniques, and computational modeling offer promising pathways forward, but integration of these approaches into standardized protocols remains an ongoing challenge that requires coordinated effort across the scientific community.
Extraction and isolation procedures introduce another layer of complexity, as different protocols can selectively isolate specific FA fractions, potentially altering the native properties of the original material. The lack of universally accepted extraction standards means that comparisons between studies often involve samples prepared under divergent conditions, raising questions about data comparability and reproducibility.
Analytical limitations further compound these challenges. Traditional techniques such as elemental analysis provide only bulk properties, while more advanced methods like nuclear magnetic resonance (NMR) spectroscopy often yield complex spectra that are difficult to interpret definitively. High-resolution mass spectrometry, while powerful, captures only ionizable components and may miss significant portions of the FA composition.
Environmental factors introduce additional variables, as the physicochemical properties of FAs can be significantly influenced by pH, ionic strength, temperature, and the presence of metal ions. These contextual dependencies mean that properties measured under laboratory conditions may not accurately reflect behavior in natural or application environments.
The absence of reference standards represents perhaps the most critical challenge. Unlike pure compounds, no universal fulvic acid standard exists against which samples can be calibrated. This fundamental limitation undermines efforts to establish quantitative relationships between structural features and functional properties across different FA sources.
Interdisciplinary barriers further complicate the field, as researchers from soil science, aquatic chemistry, and commercial applications often employ different terminologies and methodological approaches. This fragmentation of knowledge impedes the development of comprehensive characterization frameworks that could facilitate meaningful comparisons.
Recent technological advances in multi-dimensional chromatography, advanced spectroscopic techniques, and computational modeling offer promising pathways forward, but integration of these approaches into standardized protocols remains an ongoing challenge that requires coordinated effort across the scientific community.
Comparative Analysis Methodologies
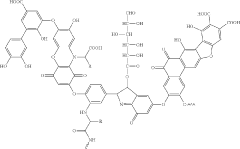
01 Chemical structure and composition of fulvic acid
Fulvic acid is characterized by its complex molecular structure containing various functional groups including carboxyl, phenolic hydroxyl, and carbonyl groups. It has a lower molecular weight compared to humic acid, typically ranging from 500 to 2000 Da. The chemical composition includes carbon, hydrogen, oxygen, and nitrogen in varying proportions, with a high oxygen content relative to carbon. This unique structure contributes to its high solubility across different pH ranges and its ability to form complexes with various elements.- Chemical structure and composition of fulvic acid: Fulvic acid is a complex organic compound with a heterogeneous structure containing various functional groups including carboxyl, phenolic hydroxyl, and carbonyl groups. It has a relatively low molecular weight compared to other humic substances, typically ranging from 500 to 2000 Da. The chemical composition includes carbon, hydrogen, oxygen, and nitrogen in varying proportions, with high oxygen content in the form of functional groups that contribute to its high solubility and reactivity.
- Solubility and pH-dependent behavior: Fulvic acid exhibits high solubility in water across a wide pH range, which distinguishes it from other humic substances. Its solubility is attributed to the presence of numerous oxygen-containing functional groups. The acid shows pH-dependent behavior, with its properties changing based on environmental pH. At lower pH values, fulvic acid tends to form more compact structures, while at higher pH values, it adopts more extended conformations due to the repulsion between negatively charged groups.
- Chelation and metal-binding properties: One of the most significant physicochemical properties of fulvic acid is its strong chelation capacity. It can form stable complexes with various metal ions including iron, copper, zinc, and calcium through its carboxyl and phenolic hydroxyl groups. This metal-binding ability affects the bioavailability and mobility of metals in soils and water systems. The chelation properties depend on pH, with optimal metal binding occurring at specific pH ranges depending on the metal ion involved.
- Optical and spectroscopic characteristics: Fulvic acid exhibits distinctive optical properties, including strong absorption in the UV-visible spectrum and fluorescence behavior. It typically shows decreasing absorbance with increasing wavelength in the UV-visible range. The spectroscopic characteristics vary depending on the source and extraction method of the fulvic acid. These optical properties are often used for identification and characterization purposes, with specific fluorescence emission patterns serving as fingerprints for different fulvic acid sources.
- Redox properties and antioxidant activity: Fulvic acid possesses significant redox properties due to its electron-donating functional groups, particularly phenolic and semiquinone moieties. It can participate in redox reactions, acting as both an electron donor and acceptor depending on environmental conditions. This property contributes to its antioxidant activity, allowing it to scavenge free radicals and reduce oxidative stress. The redox potential of fulvic acid varies with pH and is influenced by the presence of metal ions, particularly transition metals that can form redox-active complexes.
02 Solubility and pH-dependent behavior
Fulvic acid exhibits high solubility in water across a wide pH range, remaining soluble even in strongly acidic conditions (pH < 2) where other humic substances precipitate. This solubility is attributed to its smaller molecular size and higher oxygen content. The acid dissociation constants (pKa) of fulvic acid typically range between 3.0 and 5.0, indicating its moderate acidity. As pH increases, the negative charge density of fulvic acid increases due to deprotonation of functional groups, affecting its binding capacity with metals and other compounds.Expand Specific Solutions03 Metal complexation and chelation properties
Fulvic acid possesses strong metal complexation and chelation properties due to its abundant functional groups, particularly carboxyl and phenolic hydroxyl groups. These properties enable it to form stable complexes with various metal ions including iron, copper, zinc, and calcium. The stability constants of these metal-fulvic acid complexes vary depending on pH, ionic strength, and the specific metal involved. This chelation ability enhances the bioavailability of essential minerals while potentially reducing the toxicity of heavy metals in environmental and biological systems.Expand Specific Solutions04 Spectroscopic characteristics and analytical methods
Fulvic acid exhibits distinctive spectroscopic properties that are used for its characterization and quantification. It shows strong UV-visible absorption with decreasing absorbance as wavelength increases, and specific fluorescence emission patterns. The E4/E6 ratio (absorbance at 465nm divided by absorbance at 665nm) is typically higher for fulvic acid compared to humic acid, indicating its lower molecular weight and aromaticity. Analytical methods for characterizing fulvic acid include FTIR spectroscopy, NMR spectroscopy, mass spectrometry, and size exclusion chromatography, which provide insights into its structural features and functional group composition.Expand Specific Solutions05 Redox properties and antioxidant activity
Fulvic acid demonstrates significant redox properties and antioxidant activity due to its electron-rich aromatic structures and functional groups. It can act as both an electron donor and acceptor, participating in various redox reactions. The redox potential of fulvic acid typically ranges from +400 to +700 mV, depending on source and environmental conditions. This property enables it to scavenge free radicals and reactive oxygen species, contributing to its antioxidant effects. The antioxidant capacity is influenced by the phenolic hydroxyl content and the degree of aromaticity in the fulvic acid structure.Expand Specific Solutions
Leading Producers and Research Institutions
The fulvic acid market is currently in a growth phase, with increasing applications across agricultural, pharmaceutical, and environmental sectors. Market size is expanding due to rising demand for organic solutions in agriculture and health supplements, estimated to reach several billion dollars globally by 2028. Technologically, the field shows varying maturity levels across applications. Leading companies like Nihon Nohyaku and ISHIHARA SANGYO KAISHA have established advanced extraction and standardization processes for agricultural applications, while pharmaceutical players such as Teva and Mannatech are exploring novel therapeutic applications. Research institutions including Nankai University and the Chinese Research Academy of Environmental Sciences are advancing characterization methodologies, while companies like Actagro and Agro Innovation International focus on enhancing bioavailability and stability of fulvic acid formulations for sustainable agriculture.
Chinese Research Academy of Environmental Sciences
Technical Solution: The Chinese Research Academy of Environmental Sciences has pioneered comprehensive comparative analysis methodologies for fulvic acids from diverse environmental sources. Their research framework incorporates multi-dimensional characterization including elemental composition, functional group distribution, and molecular weight fractionation. Their studies have revealed significant variations in fulvic acid properties based on geographical origin, with soil-derived fulvic acids showing higher aromaticity (SUVA254 values of 3.5-4.8 L/mg·m) compared to aquatic sources (1.8-2.7 L/mg·m). The Academy has developed standardized protocols for isolating fulvic acids using XAD resin techniques that achieve over 85% recovery rates while maintaining structural integrity. Their comparative analyses have established correlations between source material decomposition stage and resulting fulvic acid properties, particularly regarding oxygen-containing functional groups and metal binding capacities. The Academy's research has demonstrated that fulvic acids from different sources exhibit varying degrees of photoreactivity and redox properties, with implications for environmental fate and remediation applications.
Strengths: Comprehensive analytical capabilities spanning multiple characterization techniques; extensive database of fulvic acid properties from diverse Chinese ecosystems; strong focus on environmental applications and remediation potential. Weaknesses: Research primarily focused on environmental rather than commercial applications; limited industrial-scale processing experience; methodologies may require adaptation for commercial production.
Mannatech, Inc.
Technical Solution: Mannatech has developed proprietary technology for standardizing fulvic acid extracts for nutritional and health applications. Their approach focuses on comparative analysis of fulvic acids from various organic-rich deposits to identify sources with optimal bioactive properties. Their research has established standardized methods for quantifying key parameters including total acidity (ranging from 5.6-7.8 meq/g), functional group distribution, and trace mineral content profiles. Mannatech's comparative studies have demonstrated significant variations in antioxidant capacity between fulvic acid sources, with ORAC values ranging from 8,000-12,000 μmol TE/g depending on origin and extraction methods. Their technology includes proprietary purification processes that reduce heavy metal content while preserving bioactive components, achieving contaminant levels below 1 ppm for lead, arsenic, and mercury. Their research has established correlations between specific spectroscopic parameters (particularly UV-visible absorbance ratios) and biological activity in cell culture systems, enabling selection of optimal fulvic acid sources for nutritional applications.
Strengths: Extensive experience in standardization and quality control for human consumption applications; well-developed purification technologies for contaminant removal; established clinical research program evaluating biological effects. Weaknesses: Primarily focused on nutritional/supplement applications rather than broader industrial uses; proprietary nature of some methodologies limits scientific validation; relatively narrow source material focus compared to research institutions.
Key Patents and Scientific Literature
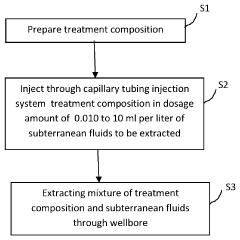
Chemical compositions and in-situ methods of using same for remediating sulfur-containing compositions and other contaminants in fluids being extracted from the earth
PatentActiveUS20230102592A1
Innovation
- An in-situ treatment method involving the injection of an aqueous-based treatment composition containing 35-55% hydroxide compounds deep into the earth, where it mixes with fluids under high pressure and temperature, effectively remediating contaminants as the fluids are extracted, utilizing the natural subterranean conditions for enhanced efficiency and cost-effectiveness.
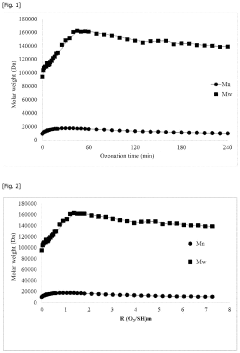
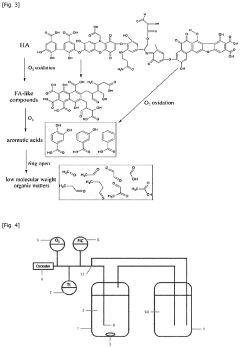
Oxidative method for preparing a fertilizing composition
PatentPendingUS20230331636A1
Innovation
- A method involving mild oxidation of humic substances using an appropriate amount of oxidizing agents, such as ozone, to increase the weight-average hydrodynamic radius of particles in solution, measured by Dynamic Light Scattering (DLS), thereby improving the fertilizing properties of the composition.
Environmental Impact of Extraction Processes
The extraction processes used to obtain fulvic acids from various sources have significant environmental implications that warrant careful consideration. Traditional extraction methods often involve the use of strong alkaline solutions, followed by acidification steps that generate substantial amounts of waste. These processes typically consume large volumes of water and chemicals, contributing to environmental pollution when not properly managed. For instance, the extraction of fulvic acids from coal using sodium hydroxide produces effluents with high pH levels that require neutralization before disposal.
Different source materials necessitate varying extraction intensities, with coal-derived fulvic acids generally requiring more aggressive chemical treatments compared to soil or compost sources. This translates to a higher environmental footprint for coal-based extraction processes. The energy consumption associated with these extraction methods also differs significantly, with coal processing typically demanding more energy for grinding, heating, and purification stages.
Water usage represents another critical environmental concern. Soil and peat-based extraction processes may require multiple washing steps, consuming substantial water resources. In contrast, more modern extraction techniques from plant materials can be designed with water recycling systems, reducing overall consumption. The potential for implementing closed-loop systems varies considerably depending on the source material and extraction technology employed.
Chemical waste generation constitutes perhaps the most direct environmental impact. The acidification step in traditional extraction protocols produces salt by-products that must be properly disposed of or repurposed. Extraction from oxidized coal can generate sulfur-containing compounds that pose additional environmental risks if released untreated. Conversely, extraction from composted materials typically produces fewer hazardous by-products, though still requires careful waste management practices.
Recent advancements have focused on developing greener extraction methodologies. These include subcritical water extraction, which reduces chemical usage, and enzymatic extraction approaches that operate under milder conditions. Such innovations show promising reductions in environmental impact, particularly for plant and compost-derived fulvic acids. However, these methods often yield products with somewhat different physicochemical properties compared to those obtained through conventional extraction, highlighting the complex relationship between extraction methodology, product characteristics, and environmental considerations.
Carbon footprint assessments of various extraction processes reveal that source material selection significantly influences overall environmental impact. Life cycle analyses indicate that locally sourced organic materials generally offer lower transportation-related emissions compared to coal-based sources, which may require long-distance shipping from mining regions.
Different source materials necessitate varying extraction intensities, with coal-derived fulvic acids generally requiring more aggressive chemical treatments compared to soil or compost sources. This translates to a higher environmental footprint for coal-based extraction processes. The energy consumption associated with these extraction methods also differs significantly, with coal processing typically demanding more energy for grinding, heating, and purification stages.
Water usage represents another critical environmental concern. Soil and peat-based extraction processes may require multiple washing steps, consuming substantial water resources. In contrast, more modern extraction techniques from plant materials can be designed with water recycling systems, reducing overall consumption. The potential for implementing closed-loop systems varies considerably depending on the source material and extraction technology employed.
Chemical waste generation constitutes perhaps the most direct environmental impact. The acidification step in traditional extraction protocols produces salt by-products that must be properly disposed of or repurposed. Extraction from oxidized coal can generate sulfur-containing compounds that pose additional environmental risks if released untreated. Conversely, extraction from composted materials typically produces fewer hazardous by-products, though still requires careful waste management practices.
Recent advancements have focused on developing greener extraction methodologies. These include subcritical water extraction, which reduces chemical usage, and enzymatic extraction approaches that operate under milder conditions. Such innovations show promising reductions in environmental impact, particularly for plant and compost-derived fulvic acids. However, these methods often yield products with somewhat different physicochemical properties compared to those obtained through conventional extraction, highlighting the complex relationship between extraction methodology, product characteristics, and environmental considerations.
Carbon footprint assessments of various extraction processes reveal that source material selection significantly influences overall environmental impact. Life cycle analyses indicate that locally sourced organic materials generally offer lower transportation-related emissions compared to coal-based sources, which may require long-distance shipping from mining regions.
Standardization and Quality Control Measures
The standardization and quality control of fulvic acid sources represents a critical challenge in both research and commercial applications. Current methodologies exhibit significant variability across laboratories and manufacturers, leading to inconsistent product quality and research outcomes. Establishing robust standardization protocols requires multi-parameter assessment frameworks that account for elemental composition, functional group distribution, molecular weight profiles, and bioactivity markers.
International organizations including the International Humic Substances Society (IHSS) have developed reference materials and analytical protocols, though adoption remains inconsistent across sectors. These standards typically specify acceptable ranges for carbon content (45-60%), oxygen content (30-45%), hydrogen content (3-6%), and nitrogen content (1-4%), alongside functional group distributions and E4/E6 ratios between 6.0-8.5 for high-quality fulvic acids.
Quality control measures must incorporate advanced analytical techniques including 13C-NMR spectroscopy, pyrolysis-GC/MS, and high-resolution mass spectrometry to verify structural characteristics. Batch-to-batch consistency monitoring through statistical process control charts has emerged as an industry best practice, with control limits typically set at ±10% for key parameters including humic-to-fulvic acid ratios and total organic carbon content.
Certification programs have developed in response to market demands for verified products, with third-party verification systems now available through organizations such as NSF International and the Organic Materials Review Institute. These certification pathways typically require comprehensive documentation of extraction methods, purification protocols, and regular analytical testing regimens.
Emerging technologies for rapid quality assessment include portable spectroscopic devices utilizing near-infrared and fluorescence spectroscopy, enabling field-based quality verification. These technologies demonstrate 85-95% correlation with laboratory methods while reducing analysis time from days to minutes, though sensitivity limitations remain for trace contaminant detection.
Harmonization efforts between regulatory frameworks in North America, Europe, and Asia represent a significant opportunity for establishing global standards. The development of international reference materials specifically for fulvic acid characterization would substantially improve cross-laboratory comparability and product consistency. Current initiatives through ISO Technical Committee 190 aim to address these standardization gaps within the next three years.
International organizations including the International Humic Substances Society (IHSS) have developed reference materials and analytical protocols, though adoption remains inconsistent across sectors. These standards typically specify acceptable ranges for carbon content (45-60%), oxygen content (30-45%), hydrogen content (3-6%), and nitrogen content (1-4%), alongside functional group distributions and E4/E6 ratios between 6.0-8.5 for high-quality fulvic acids.
Quality control measures must incorporate advanced analytical techniques including 13C-NMR spectroscopy, pyrolysis-GC/MS, and high-resolution mass spectrometry to verify structural characteristics. Batch-to-batch consistency monitoring through statistical process control charts has emerged as an industry best practice, with control limits typically set at ±10% for key parameters including humic-to-fulvic acid ratios and total organic carbon content.
Certification programs have developed in response to market demands for verified products, with third-party verification systems now available through organizations such as NSF International and the Organic Materials Review Institute. These certification pathways typically require comprehensive documentation of extraction methods, purification protocols, and regular analytical testing regimens.
Emerging technologies for rapid quality assessment include portable spectroscopic devices utilizing near-infrared and fluorescence spectroscopy, enabling field-based quality verification. These technologies demonstrate 85-95% correlation with laboratory methods while reducing analysis time from days to minutes, though sensitivity limitations remain for trace contaminant detection.
Harmonization efforts between regulatory frameworks in North America, Europe, and Asia represent a significant opportunity for establishing global standards. The development of international reference materials specifically for fulvic acid characterization would substantially improve cross-laboratory comparability and product consistency. Current initiatives through ISO Technical Committee 190 aim to address these standardization gaps within the next three years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!