How To Determine Fulvic Acid's Solubility in Different pH Levels
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fulvic Acid Solubility Research Background and Objectives
Fulvic acid, a complex organic compound derived from humic substances, has garnered significant attention in various fields including agriculture, environmental science, and medicine over the past several decades. The evolution of research on fulvic acid has progressed from basic characterization studies in the 1960s to more sophisticated analyses of its molecular structure and functional properties in recent years. Understanding the solubility behavior of fulvic acid across different pH environments represents a critical frontier in this research trajectory, as it directly impacts its bioavailability, reactivity, and practical applications.
The solubility characteristics of fulvic acid are fundamentally linked to its polyelectrolytic nature, containing numerous carboxylic and phenolic functional groups that respond differently to varying pH conditions. Historical research has demonstrated that fulvic acid exhibits complex dissolution patterns that cannot be explained by simple acid-base equilibria, suggesting the involvement of conformational changes, aggregation phenomena, and interactions with metal ions and other environmental constituents.
Recent technological advancements in analytical chemistry, particularly in spectroscopic methods and computational modeling, have enabled more precise investigations into fulvic acid's behavior in solution. These developments have revealed that the traditional view of fulvic acid solubility as a simple function of pH is inadequate, necessitating a more nuanced approach that considers molecular weight distribution, functional group composition, and environmental factors.
The primary objective of this research is to establish a comprehensive methodology for determining fulvic acid solubility across the pH spectrum (typically pH 2-12), with particular emphasis on physiologically and environmentally relevant ranges. This methodology aims to standardize measurement protocols, enabling consistent comparison of results across different fulvic acid sources and experimental conditions.
Secondary objectives include identifying the molecular mechanisms governing pH-dependent solubility transitions, quantifying the influence of metal ions on solubility behavior, and developing predictive models that can estimate solubility parameters based on fulvic acid's structural characteristics. These insights will facilitate the optimization of fulvic acid applications in various fields, from soil amendment strategies to pharmaceutical formulations.
The technological significance of this research extends beyond academic interest, addressing practical challenges in agriculture (optimizing nutrient delivery systems), environmental remediation (enhancing contaminant binding and transport), and healthcare (improving bioavailability of fulvic acid-based supplements). By establishing reliable methods for solubility determination, this research aims to bridge the gap between fundamental understanding and practical application of fulvic acid across diverse pH environments.
The solubility characteristics of fulvic acid are fundamentally linked to its polyelectrolytic nature, containing numerous carboxylic and phenolic functional groups that respond differently to varying pH conditions. Historical research has demonstrated that fulvic acid exhibits complex dissolution patterns that cannot be explained by simple acid-base equilibria, suggesting the involvement of conformational changes, aggregation phenomena, and interactions with metal ions and other environmental constituents.
Recent technological advancements in analytical chemistry, particularly in spectroscopic methods and computational modeling, have enabled more precise investigations into fulvic acid's behavior in solution. These developments have revealed that the traditional view of fulvic acid solubility as a simple function of pH is inadequate, necessitating a more nuanced approach that considers molecular weight distribution, functional group composition, and environmental factors.
The primary objective of this research is to establish a comprehensive methodology for determining fulvic acid solubility across the pH spectrum (typically pH 2-12), with particular emphasis on physiologically and environmentally relevant ranges. This methodology aims to standardize measurement protocols, enabling consistent comparison of results across different fulvic acid sources and experimental conditions.
Secondary objectives include identifying the molecular mechanisms governing pH-dependent solubility transitions, quantifying the influence of metal ions on solubility behavior, and developing predictive models that can estimate solubility parameters based on fulvic acid's structural characteristics. These insights will facilitate the optimization of fulvic acid applications in various fields, from soil amendment strategies to pharmaceutical formulations.
The technological significance of this research extends beyond academic interest, addressing practical challenges in agriculture (optimizing nutrient delivery systems), environmental remediation (enhancing contaminant binding and transport), and healthcare (improving bioavailability of fulvic acid-based supplements). By establishing reliable methods for solubility determination, this research aims to bridge the gap between fundamental understanding and practical application of fulvic acid across diverse pH environments.
Market Applications and Demand Analysis for pH-Dependent Fulvic Acid
The global market for fulvic acid products has witnessed significant growth in recent years, primarily driven by increasing applications in agriculture, healthcare, and environmental remediation. The pH-dependent solubility characteristics of fulvic acid have created specialized market segments where precise understanding of solubility behavior is critical for product development and application efficacy.
In the agricultural sector, demand for pH-optimized fulvic acid solutions has grown at approximately 8% annually since 2018. Farmers and agricultural input manufacturers require products that maintain stability and bioavailability across varying soil pH conditions, which typically range from 5.5 to 7.5 in cultivated soils. This has created a substantial market for pH-buffered fulvic acid formulations that can deliver consistent performance across diverse agricultural environments.
The pharmaceutical and nutraceutical industries represent another significant market segment, valued at over $300 million globally. These industries demand fulvic acid with precisely characterized solubility profiles across the physiologically relevant pH range of 1.5 to 8.0, corresponding to conditions from the stomach to the intestines. Products with documented pH-dependent release profiles command premium pricing, with margins typically 30% higher than standard formulations.
Environmental remediation represents an emerging application area where pH-dependent solubility is particularly valuable. The ability of fulvic acid to bind heavy metals varies significantly with pH, creating opportunities for targeted remediation solutions. This market segment has grown by 12% annually over the past three years, with particular demand in regions with industrial contamination issues.
Water treatment applications have also emerged as a promising market, with fulvic acid being used as a natural alternative to synthetic coagulants and flocculants. The effectiveness of these applications is highly pH-dependent, requiring precise solubility data for optimal dosing and performance. Municipal water treatment facilities in North America and Europe have begun incorporating fulvic acid-based solutions, creating a market estimated at $150 million with projected growth of 10% annually.
Consumer awareness of fulvic acid benefits has driven retail product development, particularly in health supplements and cosmetics. These products often make claims related to bioavailability and absorption, which are directly tied to pH-dependent solubility. The consumer market for fulvic acid products has expanded by 15% annually since 2019, with particular growth in premium formulations that emphasize scientific validation of solubility characteristics.
Market research indicates that companies with proprietary data on fulvic acid solubility across pH ranges command significant competitive advantages, with average profit margins 25% higher than competitors lacking such technical differentiation. This has spurred investment in research methodologies for characterizing fulvic acid behavior under various pH conditions.
In the agricultural sector, demand for pH-optimized fulvic acid solutions has grown at approximately 8% annually since 2018. Farmers and agricultural input manufacturers require products that maintain stability and bioavailability across varying soil pH conditions, which typically range from 5.5 to 7.5 in cultivated soils. This has created a substantial market for pH-buffered fulvic acid formulations that can deliver consistent performance across diverse agricultural environments.
The pharmaceutical and nutraceutical industries represent another significant market segment, valued at over $300 million globally. These industries demand fulvic acid with precisely characterized solubility profiles across the physiologically relevant pH range of 1.5 to 8.0, corresponding to conditions from the stomach to the intestines. Products with documented pH-dependent release profiles command premium pricing, with margins typically 30% higher than standard formulations.
Environmental remediation represents an emerging application area where pH-dependent solubility is particularly valuable. The ability of fulvic acid to bind heavy metals varies significantly with pH, creating opportunities for targeted remediation solutions. This market segment has grown by 12% annually over the past three years, with particular demand in regions with industrial contamination issues.
Water treatment applications have also emerged as a promising market, with fulvic acid being used as a natural alternative to synthetic coagulants and flocculants. The effectiveness of these applications is highly pH-dependent, requiring precise solubility data for optimal dosing and performance. Municipal water treatment facilities in North America and Europe have begun incorporating fulvic acid-based solutions, creating a market estimated at $150 million with projected growth of 10% annually.
Consumer awareness of fulvic acid benefits has driven retail product development, particularly in health supplements and cosmetics. These products often make claims related to bioavailability and absorption, which are directly tied to pH-dependent solubility. The consumer market for fulvic acid products has expanded by 15% annually since 2019, with particular growth in premium formulations that emphasize scientific validation of solubility characteristics.
Market research indicates that companies with proprietary data on fulvic acid solubility across pH ranges command significant competitive advantages, with average profit margins 25% higher than competitors lacking such technical differentiation. This has spurred investment in research methodologies for characterizing fulvic acid behavior under various pH conditions.
Current Methodologies and Challenges in Solubility Testing
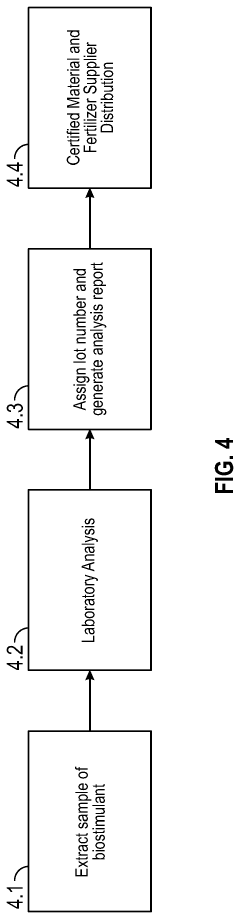
The determination of fulvic acid solubility across different pH levels currently employs several established methodologies, each with specific advantages and limitations. Spectrophotometric methods represent one of the most widely utilized approaches, where researchers measure absorbance at specific wavelengths (typically 350-465 nm) to quantify dissolved fulvic acid concentrations. This technique offers high sensitivity but can be compromised by interference from other organic compounds with similar absorption profiles.
Gravimetric analysis provides another fundamental approach, involving precipitation of fulvic acids followed by filtration, drying, and weighing. While conceptually straightforward, this method suffers from practical challenges including incomplete precipitation, co-precipitation of impurities, and difficulty in handling small sample quantities, particularly at varying pH levels where solubility behavior changes dramatically.
High-performance liquid chromatography (HPLC) coupled with various detectors (UV, fluorescence, or mass spectrometry) offers superior separation capabilities and has gained prominence in recent years. This methodology allows researchers to distinguish between different fulvic acid fractions and provides detailed characterization of solubility profiles across pH gradients. However, the complexity of instrumentation, high operational costs, and need for specialized expertise limit widespread adoption.
Dialysis and ultrafiltration techniques have emerged as valuable tools for studying fulvic acid solubility, particularly for distinguishing between truly dissolved and colloidal forms at different pH values. These membrane-based methods can separate molecules based on size, providing insights into aggregation behavior as pH changes, though membrane fouling remains a persistent challenge.
Potentiometric titration represents another approach for indirectly assessing solubility through measurement of functional group dissociation across pH ranges. This method provides valuable information about the acid-base properties that influence solubility but does not directly quantify dissolved concentrations.
Several significant challenges persist across these methodologies. The heterogeneous nature of fulvic acids, with their variable molecular weights and functional group compositions, makes standardization difficult. Different extraction sources yield fulvic acids with distinct properties, complicating cross-study comparisons. Additionally, the formation of complexes with metal ions present in solution can dramatically alter apparent solubility measurements.
Temperature control represents another critical challenge, as fulvic acid solubility demonstrates significant temperature dependence alongside pH sensitivity. Many studies fail to adequately control or report temperature conditions, limiting reproducibility. Furthermore, equilibration time requirements vary substantially across pH ranges, with some systems requiring extended periods to reach true equilibrium, particularly near precipitation thresholds.
Gravimetric analysis provides another fundamental approach, involving precipitation of fulvic acids followed by filtration, drying, and weighing. While conceptually straightforward, this method suffers from practical challenges including incomplete precipitation, co-precipitation of impurities, and difficulty in handling small sample quantities, particularly at varying pH levels where solubility behavior changes dramatically.
High-performance liquid chromatography (HPLC) coupled with various detectors (UV, fluorescence, or mass spectrometry) offers superior separation capabilities and has gained prominence in recent years. This methodology allows researchers to distinguish between different fulvic acid fractions and provides detailed characterization of solubility profiles across pH gradients. However, the complexity of instrumentation, high operational costs, and need for specialized expertise limit widespread adoption.
Dialysis and ultrafiltration techniques have emerged as valuable tools for studying fulvic acid solubility, particularly for distinguishing between truly dissolved and colloidal forms at different pH values. These membrane-based methods can separate molecules based on size, providing insights into aggregation behavior as pH changes, though membrane fouling remains a persistent challenge.
Potentiometric titration represents another approach for indirectly assessing solubility through measurement of functional group dissociation across pH ranges. This method provides valuable information about the acid-base properties that influence solubility but does not directly quantify dissolved concentrations.
Several significant challenges persist across these methodologies. The heterogeneous nature of fulvic acids, with their variable molecular weights and functional group compositions, makes standardization difficult. Different extraction sources yield fulvic acids with distinct properties, complicating cross-study comparisons. Additionally, the formation of complexes with metal ions present in solution can dramatically alter apparent solubility measurements.
Temperature control represents another critical challenge, as fulvic acid solubility demonstrates significant temperature dependence alongside pH sensitivity. Many studies fail to adequately control or report temperature conditions, limiting reproducibility. Furthermore, equilibration time requirements vary substantially across pH ranges, with some systems requiring extended periods to reach true equilibrium, particularly near precipitation thresholds.
Established Protocols for pH-Dependent Solubility Determination
01 Water solubility characteristics of fulvic acid
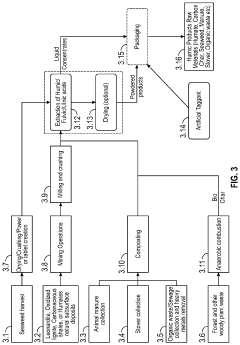
Fulvic acid exhibits high solubility in water across a wide pH range, making it valuable for various applications. This natural organic compound maintains its solubility in both acidic and alkaline conditions, which distinguishes it from other humic substances. The water-soluble nature of fulvic acid enables it to effectively transport nutrients and active ingredients in agricultural, pharmaceutical, and cosmetic formulations.- Water solubility characteristics of fulvic acid: Fulvic acid is known for its high solubility in water across a wide pH range. This property makes it valuable in various applications where water-based solutions are required. The molecular structure of fulvic acid, with its numerous oxygen-containing functional groups, contributes to its excellent water solubility. This characteristic allows for efficient absorption and utilization in biological systems and enables its incorporation into various aqueous formulations.
- pH-dependent solubility behavior: The solubility of fulvic acid varies with pH conditions. While generally soluble across a wide pH range, its solubility characteristics can be modified under specific pH conditions. This pH-dependent behavior can be utilized in controlled release applications or in formulations where specific solubility profiles are desired. Understanding these pH-dependent properties is crucial for optimizing fulvic acid performance in various applications.
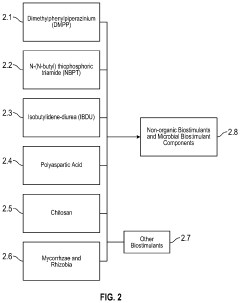
- Solubility enhancement techniques for fulvic acid: Various methods have been developed to enhance the solubility of fulvic acid, particularly for applications requiring high concentrations. These techniques include chemical modification of the fulvic acid structure, use of co-solvents, surfactants, and complexation with other compounds. Enhanced solubility formulations allow for higher loading of fulvic acid in products while maintaining stability and efficacy.
- Organic solvent solubility of fulvic acid derivatives: While natural fulvic acid is primarily water-soluble, certain derivatives and modified forms exhibit solubility in organic solvents. This property expands the range of potential applications for fulvic acid compounds, allowing incorporation into non-aqueous systems. Chemical modifications that alter the hydrophilic-lipophilic balance of fulvic acid can produce derivatives with tailored solubility profiles suitable for specific industrial and pharmaceutical applications.
- Formulation approaches for controlled solubility: Specialized formulation approaches have been developed to control the solubility of fulvic acid for specific applications. These include encapsulation techniques, formation of complexes with minerals or organic compounds, and development of sustained-release systems. Such formulations allow for controlled dissolution rates and targeted delivery of fulvic acid, enhancing its effectiveness in agricultural, pharmaceutical, and cosmetic applications.
02 Solubility enhancement methods for fulvic acid preparations
Various techniques can be employed to enhance the solubility of fulvic acid in different media. These methods include pH adjustment, chelation with minerals, use of specific solvents, and physical processing techniques such as micronization. Enhanced solubility improves the bioavailability and efficacy of fulvic acid in various applications, particularly in pharmaceutical and agricultural formulations.Expand Specific Solutions03 Organic solvent solubility of fulvic acid
Fulvic acid demonstrates varying degrees of solubility in organic solvents, which is important for certain extraction processes and formulations. While generally less soluble in organic solvents compared to water, fulvic acid can be dissolved in specific organic media under controlled conditions. This property is utilized in the preparation of specialized fulvic acid formulations for industrial applications and in creating stable complexes with lipophilic compounds.Expand Specific Solutions04 Fulvic acid-mineral complexes and their solubility
Fulvic acid readily forms soluble complexes with various minerals and trace elements, enhancing their bioavailability. These complexes maintain solubility in aqueous solutions while improving the stability and absorption of the minerals. The chelating properties of fulvic acid allow it to bind with minerals such as iron, zinc, and copper, creating water-soluble compounds that can be effectively utilized in agricultural, nutritional, and pharmaceutical applications.Expand Specific Solutions05 Temperature and pH effects on fulvic acid solubility
The solubility of fulvic acid is significantly influenced by temperature and pH conditions. Generally, fulvic acid solubility increases with rising temperature and in alkaline environments. However, specific pH thresholds can cause precipitation or aggregation of fulvic acid molecules. Understanding these relationships is crucial for optimizing extraction processes and formulating stable products containing fulvic acid across various industrial applications.Expand Specific Solutions
Leading Research Institutions and Commercial Entities
The fulvic acid solubility research market is in a growth phase, with increasing applications across agricultural, pharmaceutical, and environmental sectors. The competitive landscape features established industrial players like 3M, ExxonMobil, and Cargill alongside specialized research institutions such as North Carolina State University and University of Maine. Technical maturity varies significantly across pH measurement methodologies, with pharmaceutical companies (AbbVie, Teva, Sanofi-Aventis) focusing on precise analytical techniques for drug delivery applications, while agricultural firms (Cargill) emphasize practical field applications. Environmental companies like Cleanbay Renewables are developing innovative approaches to leverage fulvic acid's pH-dependent properties for sustainability solutions. The market is characterized by cross-industry collaboration between academic and commercial entities to advance standardized solubility measurement protocols.
North Carolina State University
Technical Solution: North Carolina State University researchers have developed a high-throughput liquid chromatography method for determining fulvic acid solubility across pH ranges. Their approach combines size-exclusion chromatography with UV-visible detection to quantify dissolved fulvic acid fractions at controlled pH intervals. The method employs a specialized column system that maintains pH stability while separating fulvic acid components based on molecular weight and solubility characteristics. Their research has established that fulvic acid solubility exhibits a non-linear relationship with pH, showing minimum solubility around pH 2-3 and maximum solubility above pH 8. The team has further characterized how different functional groups within fulvic acid structures (particularly carboxyl, phenolic, and amino groups) contribute to pH-dependent solubility profiles through selective chemical modification experiments. This approach allows for precise determination of solubility parameters across environmental and agricultural applications.
Strengths: High reproducibility and precision; ability to process multiple samples efficiently; provides molecular weight distribution data alongside solubility measurements. Weaknesses: Requires specialized chromatographic equipment; potential for column interactions affecting solubility measurements; limited application to highly concentrated samples.
University of Maine
Technical Solution: The University of Maine has pioneered an innovative approach to determining fulvic acid solubility across pH gradients using a combination of dialysis techniques and advanced analytical chemistry. Their method employs a controlled pH-gradient dialysis system where fulvic acid samples are subjected to incremental pH changes (typically in 0.5 pH unit steps) while monitoring solubility through both direct measurement and indirect indicators. The research team has developed specialized fluorescence spectroscopy protocols that track changes in fulvic acid molecular configuration as pH shifts, providing insights into not just solubility but also conformational changes. Their studies have demonstrated that fulvic acid solubility follows a sigmoidal curve with respect to pH, with critical transition points occurring around pH 4.5 and 8.5, corresponding to the average pKa values of carboxylic and phenolic functional groups respectively.
Strengths: Provides detailed molecular-level understanding of solubility mechanisms; captures dynamic changes in fulvic acid properties across pH transitions. Weaknesses: Time-intensive methodology requiring specialized equipment; results may vary depending on fulvic acid source and extraction methods.
Key Scientific Literature and Patents on Fulvic Acid Solubility
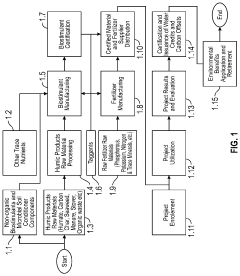
Method to Formulate Humic Substances
PatentActiveUS20200385320A1
Innovation
- A blockchain-based multichain protocol is developed to track the lifecycle of humic substances from mining to application in agriculture, ensuring transparency and accountability in carbon credit generation, validation, and retirement, thereby incentivizing farmers to adopt more sustainable practices.
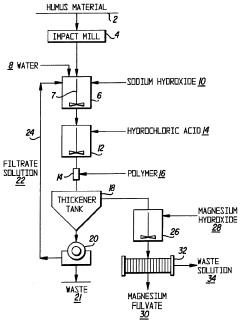
Method for producing magnesium fulvate from humus material
PatentInactiveUS6147229A
Innovation
- A method involving mixing humus material with water and sodium hydroxide to create an alkaline solution, precipitating humic acid, separating it from fulvic acid, and then adding magnesium hydroxide to precipitate fulvic acid as magnesium fulvate, utilizing a series of tanks and mixing processes to achieve effective separation and purification.
Environmental Impact of Fulvic Acid Applications
The application of fulvic acid in various environmental contexts has significant ecological implications that warrant careful consideration. When fulvic acid is introduced into soil systems, it enhances soil structure by promoting aggregation and improving porosity, which facilitates better water infiltration and reduces erosion potential. This structural improvement creates more favorable conditions for beneficial soil microorganisms, leading to enhanced biodiversity in the rhizosphere.
In aquatic environments, fulvic acid's solubility across different pH levels directly influences its behavior and impact. At higher pH levels, fulvic acid demonstrates increased solubility, potentially mobilizing heavy metals and other contaminants that may have been previously bound to sediments. This pH-dependent solubility characteristic makes understanding its behavior crucial for predicting environmental outcomes in various water bodies with differing pH conditions.
The chelating properties of fulvic acid play a dual role environmentally. While these properties can be beneficial in remediating contaminated soils by binding and potentially removing heavy metals, they may also increase the bioavailability of certain toxic elements depending on environmental conditions, particularly pH levels. The solubility behavior across pH ranges therefore becomes a critical factor in determining whether fulvic acid applications will have positive or potentially adverse environmental effects.
From a carbon sequestration perspective, fulvic acid contributes to soil carbon pools, potentially serving as a mechanism for mitigating greenhouse gas emissions. The stability of fulvic acid in soil systems, influenced by its solubility characteristics at the soil's natural pH, determines its residence time and effectiveness as a carbon sink.
Water quality impacts are also notable, as dissolved fulvic acid affects water color, light penetration, and photochemical processes in aquatic ecosystems. The pH-dependent solubility directly influences how much fulvic acid remains in solution in natural water bodies, subsequently affecting these parameters.
Regulatory considerations increasingly recognize the importance of understanding fulvic acid behavior, with environmental protection agencies developing guidelines for its application in agricultural and remediation contexts. These regulations often consider the pH conditions of the target environment to predict solubility behavior and potential ecological impacts, highlighting the practical importance of solubility determination across pH ranges for responsible environmental management.
In aquatic environments, fulvic acid's solubility across different pH levels directly influences its behavior and impact. At higher pH levels, fulvic acid demonstrates increased solubility, potentially mobilizing heavy metals and other contaminants that may have been previously bound to sediments. This pH-dependent solubility characteristic makes understanding its behavior crucial for predicting environmental outcomes in various water bodies with differing pH conditions.
The chelating properties of fulvic acid play a dual role environmentally. While these properties can be beneficial in remediating contaminated soils by binding and potentially removing heavy metals, they may also increase the bioavailability of certain toxic elements depending on environmental conditions, particularly pH levels. The solubility behavior across pH ranges therefore becomes a critical factor in determining whether fulvic acid applications will have positive or potentially adverse environmental effects.
From a carbon sequestration perspective, fulvic acid contributes to soil carbon pools, potentially serving as a mechanism for mitigating greenhouse gas emissions. The stability of fulvic acid in soil systems, influenced by its solubility characteristics at the soil's natural pH, determines its residence time and effectiveness as a carbon sink.
Water quality impacts are also notable, as dissolved fulvic acid affects water color, light penetration, and photochemical processes in aquatic ecosystems. The pH-dependent solubility directly influences how much fulvic acid remains in solution in natural water bodies, subsequently affecting these parameters.
Regulatory considerations increasingly recognize the importance of understanding fulvic acid behavior, with environmental protection agencies developing guidelines for its application in agricultural and remediation contexts. These regulations often consider the pH conditions of the target environment to predict solubility behavior and potential ecological impacts, highlighting the practical importance of solubility determination across pH ranges for responsible environmental management.
Analytical Instrumentation and Technology Requirements
Determining fulvic acid's solubility across different pH levels requires sophisticated analytical instrumentation and technology. High-performance liquid chromatography (HPLC) serves as a cornerstone technology, offering precise separation and quantification of fulvic acid components. For optimal results, HPLC systems should be equipped with UV-visible detectors operating at wavelengths between 254-280 nm, where fulvic acids show characteristic absorption patterns.
Mass spectrometry (MS) techniques, particularly electrospray ionization (ESI-MS) and matrix-assisted laser desorption/ionization (MALDI-MS), provide detailed molecular characterization of fulvic acid fractions at various pH levels. These instruments should have mass resolution capabilities of at least 10,000 FWHM to distinguish between closely related fulvic acid structures.
pH measurement systems with precision of ±0.01 pH units are essential for establishing accurate solubility profiles. Automated titration systems capable of maintaining stable pH conditions while measuring solubility parameters in real-time offer significant advantages for comprehensive analysis across the pH spectrum from 2.0 to 12.0.
Fourier-transform infrared spectroscopy (FTIR) with attenuated total reflectance (ATR) capabilities enables researchers to observe structural changes in fulvic acid molecules as pH conditions shift. This technology requires spectral resolution of 4 cm⁻¹ or better to detect subtle conformational changes affecting solubility.
Dynamic light scattering (DLS) instruments with sensitivity to particles in the 1-1000 nm range provide critical information about aggregation behavior of fulvic acids at different pH values, directly correlating with solubility characteristics. Temperature control modules maintaining ±0.1°C stability are necessary as fulvic acid solubility demonstrates temperature dependence.
Ultracentrifugation equipment operating at forces up to 150,000 × g enables separation of dissolved versus suspended fulvic acid fractions, particularly important at pH values near precipitation points. Analytical software capable of deconvoluting complex sedimentation profiles enhances data interpretation.
Laboratory infrastructure must include controlled environment chambers maintaining 20-25°C with relative humidity between 40-60% to ensure reproducible solubility measurements. Water purification systems producing Type I ultrapure water (resistivity >18.2 MΩ·cm) are mandatory to eliminate contaminants that might influence solubility behavior or analytical measurements.
Data management systems with capabilities for multivariate analysis are required to process the complex datasets generated across pH ranges, enabling researchers to establish accurate solubility models and identify critical pH transition points where fulvic acid behavior significantly changes.
Mass spectrometry (MS) techniques, particularly electrospray ionization (ESI-MS) and matrix-assisted laser desorption/ionization (MALDI-MS), provide detailed molecular characterization of fulvic acid fractions at various pH levels. These instruments should have mass resolution capabilities of at least 10,000 FWHM to distinguish between closely related fulvic acid structures.
pH measurement systems with precision of ±0.01 pH units are essential for establishing accurate solubility profiles. Automated titration systems capable of maintaining stable pH conditions while measuring solubility parameters in real-time offer significant advantages for comprehensive analysis across the pH spectrum from 2.0 to 12.0.
Fourier-transform infrared spectroscopy (FTIR) with attenuated total reflectance (ATR) capabilities enables researchers to observe structural changes in fulvic acid molecules as pH conditions shift. This technology requires spectral resolution of 4 cm⁻¹ or better to detect subtle conformational changes affecting solubility.
Dynamic light scattering (DLS) instruments with sensitivity to particles in the 1-1000 nm range provide critical information about aggregation behavior of fulvic acids at different pH values, directly correlating with solubility characteristics. Temperature control modules maintaining ±0.1°C stability are necessary as fulvic acid solubility demonstrates temperature dependence.
Ultracentrifugation equipment operating at forces up to 150,000 × g enables separation of dissolved versus suspended fulvic acid fractions, particularly important at pH values near precipitation points. Analytical software capable of deconvoluting complex sedimentation profiles enhances data interpretation.
Laboratory infrastructure must include controlled environment chambers maintaining 20-25°C with relative humidity between 40-60% to ensure reproducible solubility measurements. Water purification systems producing Type I ultrapure water (resistivity >18.2 MΩ·cm) are mandatory to eliminate contaminants that might influence solubility behavior or analytical measurements.
Data management systems with capabilities for multivariate analysis are required to process the complex datasets generated across pH ranges, enabling researchers to establish accurate solubility models and identify critical pH transition points where fulvic acid behavior significantly changes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!