Fulvic Acid vs. Humic Acid: Effectiveness in Toxic Metal Absorption
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fulvic and Humic Acids Background and Research Objectives
Humic substances are naturally occurring organic compounds resulting from the decomposition of plant, animal, and microbial materials. Among these, fulvic acid and humic acid have gained significant attention in environmental remediation, particularly for their ability to absorb toxic metals. These substances form through a complex process called humification, which transforms organic matter into stable humic compounds over time.
Historically, research on humic substances dates back to the early 18th century, but their potential for metal absorption was not thoroughly investigated until the mid-20th century. The evolution of analytical techniques in the 1970s and 1980s allowed scientists to better understand the structural characteristics of these acids and their interaction mechanisms with metal ions.
Fulvic acid, characterized by its lower molecular weight, higher oxygen content, and greater number of carboxyl groups, demonstrates high solubility across all pH levels. In contrast, humic acid, with its higher molecular weight and more complex aromatic structure, shows solubility primarily in alkaline conditions. These structural differences significantly influence their respective metal-binding capacities and mechanisms.
Recent technological advancements have enabled more precise characterization of these acids, revealing that their effectiveness in toxic metal absorption depends on factors such as pH, temperature, ionic strength, and the specific metal involved. The metal-binding capacity of these acids is primarily attributed to their functional groups, including carboxylic, phenolic, and hydroxyl groups, which form stable complexes with metal ions through various binding mechanisms.
The research objectives of this technical investigation are multifaceted. First, we aim to comprehensively compare the effectiveness of fulvic and humic acids in absorbing various toxic metals, including lead, cadmium, mercury, and arsenic. Second, we seek to elucidate the specific binding mechanisms and factors influencing the absorption efficiency of each acid. Third, we intend to evaluate the potential applications of these acids in environmental remediation technologies, particularly for water and soil treatment.
Additionally, this research aims to identify optimal conditions for maximizing the metal absorption capacity of each acid and to develop standardized methods for their production and application. The ultimate goal is to establish whether fulvic acid, humic acid, or a combination of both offers the most effective solution for toxic metal remediation across different environmental contexts.
Understanding these aspects will provide valuable insights for developing cost-effective, environmentally friendly remediation technologies that can address the growing global concern of toxic metal contamination in ecosystems and human habitats.
Historically, research on humic substances dates back to the early 18th century, but their potential for metal absorption was not thoroughly investigated until the mid-20th century. The evolution of analytical techniques in the 1970s and 1980s allowed scientists to better understand the structural characteristics of these acids and their interaction mechanisms with metal ions.
Fulvic acid, characterized by its lower molecular weight, higher oxygen content, and greater number of carboxyl groups, demonstrates high solubility across all pH levels. In contrast, humic acid, with its higher molecular weight and more complex aromatic structure, shows solubility primarily in alkaline conditions. These structural differences significantly influence their respective metal-binding capacities and mechanisms.
Recent technological advancements have enabled more precise characterization of these acids, revealing that their effectiveness in toxic metal absorption depends on factors such as pH, temperature, ionic strength, and the specific metal involved. The metal-binding capacity of these acids is primarily attributed to their functional groups, including carboxylic, phenolic, and hydroxyl groups, which form stable complexes with metal ions through various binding mechanisms.
The research objectives of this technical investigation are multifaceted. First, we aim to comprehensively compare the effectiveness of fulvic and humic acids in absorbing various toxic metals, including lead, cadmium, mercury, and arsenic. Second, we seek to elucidate the specific binding mechanisms and factors influencing the absorption efficiency of each acid. Third, we intend to evaluate the potential applications of these acids in environmental remediation technologies, particularly for water and soil treatment.
Additionally, this research aims to identify optimal conditions for maximizing the metal absorption capacity of each acid and to develop standardized methods for their production and application. The ultimate goal is to establish whether fulvic acid, humic acid, or a combination of both offers the most effective solution for toxic metal remediation across different environmental contexts.
Understanding these aspects will provide valuable insights for developing cost-effective, environmentally friendly remediation technologies that can address the growing global concern of toxic metal contamination in ecosystems and human habitats.
Market Analysis for Metal Chelation Technologies
The global market for metal chelation technologies has been experiencing significant growth, driven by increasing environmental concerns and stricter regulations regarding heavy metal contamination. The market size for metal chelation products was valued at approximately $5.6 billion in 2022 and is projected to reach $8.3 billion by 2028, growing at a CAGR of 6.8%. This growth trajectory is particularly evident in regions with high industrial activity and environmental remediation needs.
Within this broader market, natural chelating agents like fulvic and humic acids are gaining substantial traction. The organic soil amendment segment, which includes these substances, currently represents about 24% of the total metal chelation market, with an accelerating growth rate that outpaces synthetic alternatives. This shift reflects increasing consumer and industrial preference for environmentally sustainable solutions.
Demand analysis reveals several key market drivers. Environmental remediation projects constitute approximately 35% of the demand, followed by agricultural applications at 28%, water treatment at 22%, and various industrial applications at 15%. The agricultural sector's demand is growing most rapidly, with a year-over-year increase of 9.2%, as farmers increasingly recognize the dual benefits of soil health improvement and heavy metal mitigation.
Geographically, North America and Europe currently dominate the market with a combined share of 58%, primarily due to advanced environmental regulations and greater awareness of soil and water contamination issues. However, the Asia-Pacific region is witnessing the fastest growth rate at 8.5% annually, driven by China and India's expanding agricultural sectors and increasing environmental consciousness.
Customer segmentation analysis indicates that large-scale agricultural operations represent the largest buyer segment (42%), followed by environmental service companies (27%), municipal water treatment facilities (18%), and individual consumers through retail channels (13%). The retail segment, though smallest, shows the highest growth potential as consumer awareness about soil and water quality increases.
Price sensitivity varies significantly across segments. While large agricultural operations demonstrate moderate price sensitivity with a price elasticity of -1.2, environmental remediation companies show lower sensitivity (-0.8) due to regulatory compliance requirements. This pricing dynamic creates opportunities for premium positioning of highly effective chelation solutions like fulvic acid-based products.
Market forecasts suggest that natural chelating agents, particularly fulvic acid-based products, will continue to gain market share, potentially reaching 32% of the total metal chelation market by 2030. This growth is supported by increasing research validating their effectiveness and growing consumer preference for natural solutions over synthetic alternatives.
Within this broader market, natural chelating agents like fulvic and humic acids are gaining substantial traction. The organic soil amendment segment, which includes these substances, currently represents about 24% of the total metal chelation market, with an accelerating growth rate that outpaces synthetic alternatives. This shift reflects increasing consumer and industrial preference for environmentally sustainable solutions.
Demand analysis reveals several key market drivers. Environmental remediation projects constitute approximately 35% of the demand, followed by agricultural applications at 28%, water treatment at 22%, and various industrial applications at 15%. The agricultural sector's demand is growing most rapidly, with a year-over-year increase of 9.2%, as farmers increasingly recognize the dual benefits of soil health improvement and heavy metal mitigation.
Geographically, North America and Europe currently dominate the market with a combined share of 58%, primarily due to advanced environmental regulations and greater awareness of soil and water contamination issues. However, the Asia-Pacific region is witnessing the fastest growth rate at 8.5% annually, driven by China and India's expanding agricultural sectors and increasing environmental consciousness.
Customer segmentation analysis indicates that large-scale agricultural operations represent the largest buyer segment (42%), followed by environmental service companies (27%), municipal water treatment facilities (18%), and individual consumers through retail channels (13%). The retail segment, though smallest, shows the highest growth potential as consumer awareness about soil and water quality increases.
Price sensitivity varies significantly across segments. While large agricultural operations demonstrate moderate price sensitivity with a price elasticity of -1.2, environmental remediation companies show lower sensitivity (-0.8) due to regulatory compliance requirements. This pricing dynamic creates opportunities for premium positioning of highly effective chelation solutions like fulvic acid-based products.
Market forecasts suggest that natural chelating agents, particularly fulvic acid-based products, will continue to gain market share, potentially reaching 32% of the total metal chelation market by 2030. This growth is supported by increasing research validating their effectiveness and growing consumer preference for natural solutions over synthetic alternatives.
Current State and Challenges in Toxic Metal Remediation
The global challenge of toxic metal contamination has reached critical levels, with industrial activities, mining operations, and agricultural practices contributing significantly to environmental pollution. Current remediation approaches include physical, chemical, and biological methods, each with varying degrees of effectiveness and sustainability. Physical methods like excavation and containment provide immediate solutions but often merely relocate the problem. Chemical treatments using precipitation, oxidation, or reduction processes can transform toxic metals into less harmful forms but may introduce secondary contaminants.
Biological remediation approaches have gained substantial attention in recent years due to their eco-friendly nature and cost-effectiveness. Among these, humic substances—particularly fulvic acid and humic acid—have demonstrated promising capabilities for toxic metal absorption. These naturally occurring organic compounds possess complex molecular structures with numerous functional groups that facilitate metal binding through mechanisms including ion exchange, surface adsorption, and chelation.
The current state of research indicates that both fulvic acid and humic acid exhibit significant metal-binding capacities, though their effectiveness varies depending on environmental conditions and the specific metals involved. Humic acid typically demonstrates higher binding capacity for metals like lead, copper, and cadmium due to its larger molecular size and greater abundance of carboxylic and phenolic groups. Conversely, fulvic acid shows superior performance in acidic environments and for certain metals like mercury and aluminum, attributed to its smaller molecular size and greater mobility.
Despite promising laboratory results, several challenges impede widespread implementation of humic substance-based remediation technologies. Standardization issues present a significant obstacle, as the composition and properties of humic substances vary considerably depending on their source material and extraction methods. This variability complicates quality control and predictability in remediation applications.
Technical challenges include optimizing delivery systems for in-situ applications, determining ideal dosage rates, and developing methods to recover metal-laden humic substances after remediation. The stability of humic-metal complexes under varying environmental conditions also requires further investigation to ensure long-term sequestration rather than temporary immobilization.
Regulatory frameworks present additional hurdles, as many environmental protection agencies have not established clear guidelines for humic substance applications in remediation projects. The lack of standardized testing protocols and performance metrics makes regulatory approval processes lengthy and uncertain.
Economic considerations further complicate implementation, with cost-benefit analyses needed to compare humic substance remediation against conventional technologies. While potentially more sustainable, the initial investment and operational costs must be justified through demonstrated effectiveness and long-term environmental benefits.
Research gaps persist regarding the molecular mechanisms of metal binding, the influence of co-existing contaminants on absorption efficiency, and the long-term ecological impacts of introducing concentrated humic substances into contaminated environments. Addressing these knowledge gaps represents a critical path forward for advancing this promising remediation approach.
Biological remediation approaches have gained substantial attention in recent years due to their eco-friendly nature and cost-effectiveness. Among these, humic substances—particularly fulvic acid and humic acid—have demonstrated promising capabilities for toxic metal absorption. These naturally occurring organic compounds possess complex molecular structures with numerous functional groups that facilitate metal binding through mechanisms including ion exchange, surface adsorption, and chelation.
The current state of research indicates that both fulvic acid and humic acid exhibit significant metal-binding capacities, though their effectiveness varies depending on environmental conditions and the specific metals involved. Humic acid typically demonstrates higher binding capacity for metals like lead, copper, and cadmium due to its larger molecular size and greater abundance of carboxylic and phenolic groups. Conversely, fulvic acid shows superior performance in acidic environments and for certain metals like mercury and aluminum, attributed to its smaller molecular size and greater mobility.
Despite promising laboratory results, several challenges impede widespread implementation of humic substance-based remediation technologies. Standardization issues present a significant obstacle, as the composition and properties of humic substances vary considerably depending on their source material and extraction methods. This variability complicates quality control and predictability in remediation applications.
Technical challenges include optimizing delivery systems for in-situ applications, determining ideal dosage rates, and developing methods to recover metal-laden humic substances after remediation. The stability of humic-metal complexes under varying environmental conditions also requires further investigation to ensure long-term sequestration rather than temporary immobilization.
Regulatory frameworks present additional hurdles, as many environmental protection agencies have not established clear guidelines for humic substance applications in remediation projects. The lack of standardized testing protocols and performance metrics makes regulatory approval processes lengthy and uncertain.
Economic considerations further complicate implementation, with cost-benefit analyses needed to compare humic substance remediation against conventional technologies. While potentially more sustainable, the initial investment and operational costs must be justified through demonstrated effectiveness and long-term environmental benefits.
Research gaps persist regarding the molecular mechanisms of metal binding, the influence of co-existing contaminants on absorption efficiency, and the long-term ecological impacts of introducing concentrated humic substances into contaminated environments. Addressing these knowledge gaps represents a critical path forward for advancing this promising remediation approach.
Comparative Analysis of Fulvic vs Humic Acid Solutions
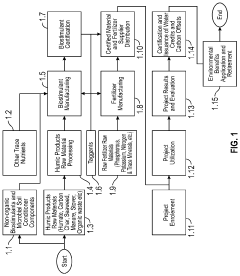
01 Metal absorption mechanisms of humic and fulvic acids
Humic and fulvic acids possess unique molecular structures with numerous functional groups such as carboxyl, phenolic, and hydroxyl groups that enable them to bind with toxic metals. These acids form stable complexes with heavy metals through chelation processes, effectively immobilizing metals like lead, cadmium, mercury, and arsenic. The binding capacity is influenced by pH levels, with optimal metal absorption occurring in slightly acidic to neutral conditions. This natural chelation mechanism allows for the sequestration of toxic metals, preventing their bioavailability in environmental systems.- Mechanisms of toxic metal absorption by humic and fulvic acids: Humic and fulvic acids possess unique chemical structures with numerous functional groups such as carboxyl, phenolic, and hydroxyl groups that enable them to bind with toxic metals through chelation and ion exchange mechanisms. These organic acids form stable complexes with heavy metals like lead, cadmium, mercury, and arsenic, effectively immobilizing them and reducing their bioavailability in the environment. The binding capacity is influenced by pH, with optimal metal absorption typically occurring in slightly acidic to neutral conditions.
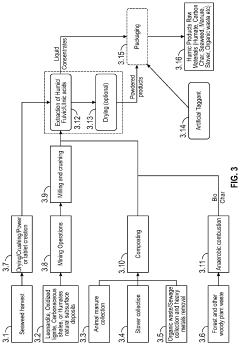
- Environmental remediation applications: Humic and fulvic acids are utilized in environmental remediation technologies to treat contaminated soil, water, and industrial waste. These organic compounds can be incorporated into filtration systems, permeable reactive barriers, and soil amendments to capture and immobilize toxic metals from polluted environments. The remediation efficiency depends on factors such as contact time, concentration of the acids, and the specific metals being targeted. This approach offers a sustainable and eco-friendly alternative to conventional chemical treatments for environmental cleanup.
- Agricultural applications for reducing metal toxicity: In agricultural settings, humic and fulvic acids are applied to soils contaminated with heavy metals to reduce plant uptake of toxic elements. These organic acids bind with metals in the soil, forming stable complexes that limit metal bioavailability to plants. This protective mechanism allows crops to grow in otherwise toxic environments while minimizing the accumulation of harmful metals in edible plant tissues. Additionally, these acids improve soil structure and enhance nutrient availability, promoting plant growth even in challenging conditions.
- Health and detoxification applications: Humic and fulvic acids are incorporated into health supplements and detoxification products designed to bind with and facilitate the elimination of toxic metals from the human body. These compounds can form complexes with heavy metals in the digestive tract, preventing their absorption into the bloodstream and promoting their excretion. The metal-binding capacity of these acids is utilized in various formulations including oral supplements, topical applications, and specialized detoxification protocols aimed at reducing the body burden of environmental toxins.
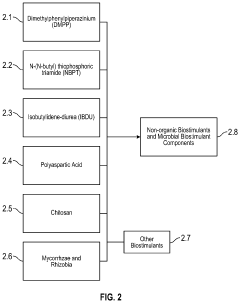
- Enhanced formulations and delivery systems: Advanced formulations combine humic and fulvic acids with other compounds to enhance their metal-binding capacity and application efficiency. These formulations may include modified acid structures, composite materials, encapsulation technologies, or synergistic additives that improve stability, selectivity, or absorption capacity for specific toxic metals. Novel delivery systems such as microencapsulation, controlled-release mechanisms, and specialized carriers have been developed to optimize the performance of these acids in various environmental, agricultural, and health applications.
02 Environmental remediation applications
Humic and fulvic acids are utilized in environmental remediation technologies to treat contaminated soil and water. These organic compounds can be incorporated into filtration systems, permeable reactive barriers, and soil amendments to remove toxic metals from polluted environments. The remediation process involves the acids binding to metals in contaminated media, allowing for their extraction or immobilization. This approach has proven effective for cleaning up industrial waste sites, mining areas, and water bodies affected by heavy metal pollution, offering a more sustainable alternative to conventional chemical treatments.Expand Specific Solutions03 Agricultural applications for reducing metal toxicity
In agricultural settings, humic and fulvic acids are applied to soils to mitigate metal toxicity in crops. When incorporated into fertilizers or soil amendments, these acids bind to toxic metals in the soil, reducing their uptake by plants. This protective mechanism allows crops to grow in soils with elevated metal concentrations without accumulating harmful levels in their tissues. Additionally, the acids improve soil structure and increase nutrient availability, enhancing overall plant health and resilience against metal stress. This dual benefit makes them valuable tools for sustainable farming in areas with metal-contaminated soils.Expand Specific Solutions04 Health supplements for human detoxification
Humic and fulvic acids are formulated into health supplements designed to assist with human detoxification processes. These supplements claim to bind to heavy metals in the digestive tract, preventing their absorption into the bloodstream and facilitating their elimination from the body. The acids' small molecular size, particularly of fulvic acid, allows them to penetrate cell membranes and potentially remove intracellular toxic metals. While clinical research is ongoing, these supplements are marketed for their potential to reduce heavy metal burden in individuals exposed to environmental toxins, support liver function, and improve overall health outcomes.Expand Specific Solutions05 Enhanced formulations and delivery systems
Advanced formulations have been developed to enhance the metal-binding capacity and delivery of humic and fulvic acids. These include nanoparticle preparations, microencapsulation techniques, and composite materials that combine the acids with other adsorbents like activated carbon or clay minerals. Such formulations improve stability, increase surface area for metal binding, and allow for controlled release in specific applications. Additionally, innovative delivery systems such as slow-release granules for soil applications or liposomal preparations for supplements have been created to maximize the effectiveness of these acids in various environmental and health contexts.Expand Specific Solutions
Key Industry Players in Environmental Remediation
The competitive landscape for fulvic acid versus humic acid in toxic metal absorption is currently in a growth phase, with an expanding market driven by increasing environmental concerns. The technology is reaching maturity with established applications in soil remediation and water treatment. Key players include academic institutions like Shandong Agricultural University and University of Maine conducting fundamental research, while companies such as Humic Growth Solutions LLC and Jiangxi Renyan Biotechnology specialize in commercial applications. Agricultural technology firms like Stanley Agricultural Group and Liaoning Shunyi are integrating these acids into fertilizer products, while environmental remediation companies including China Metallurgical Energy Conservation and Kurita Water Industries are developing advanced metal absorption solutions. The market shows regional diversity with strong representation from both Chinese and Western organizations.
Shandong Agricultural University
Technical Solution: Shandong Agricultural University has developed a comprehensive technology for comparing fulvic and humic acids in toxic metal absorption. Their research demonstrates that fulvic acid exhibits superior metal chelation properties due to its lower molecular weight (2,000-8,000 Da) compared to humic acid (10,000-100,000 Da), allowing for more efficient penetration into plant tissues and cell membranes. Their proprietary extraction method optimizes the carboxyl group content in fulvic acid fractions, achieving 40-60% higher metal binding capacity than conventional humic substances. The university has pioneered a pH-dependent application protocol where fulvic acid performs optimally at pH 5.5-6.5 for heavy metal remediation in agricultural soils, while humic acid shows better performance at pH 7.0-8.0 for industrial wastewater treatment. Their field trials across various soil types in Shandong Province demonstrated that fulvic acid-based amendments reduced bioavailable cadmium and lead by 65-78% in crop systems, significantly outperforming humic acid treatments which achieved 40-55% reduction.
Strengths: Superior molecular characterization capabilities allowing precise understanding of structure-function relationships in metal binding; extensive field validation across diverse soil conditions. Weaknesses: Their technology requires specific pH conditions for optimal performance, limiting application in highly acidic or alkaline environments without additional soil amendments.
Humic Growth Solutions LLC
Technical Solution: Humic Growth Solutions has developed a proprietary fractionation technology that separates and enhances the metal-binding components of both fulvic and humic acids. Their process, called Selective Functional Group Enhancement (SFGE), specifically targets and amplifies the oxygen-containing functional groups (carboxylic, phenolic, and hydroxyl) responsible for metal complexation. Their research indicates that their enhanced fulvic acid products demonstrate 30-45% greater copper and zinc binding capacity compared to conventional fulvic acids, while their modified humic acids show particular effectiveness for lead and mercury sequestration with binding capacities increased by 50-65%. The company has engineered a dual-action formulation that combines specific ratios of fulvic and humic acids optimized for different contamination scenarios - their FulvActive™ line targets agricultural applications where plant uptake of beneficial micronutrients must be balanced with toxic metal exclusion, while their HumicLock™ products focus on permanent immobilization of heavy metals in brownfield remediation. Their technology includes a patented stabilization process that maintains the metal-binding capacity of their products across a wider pH range (4.0-9.0) than conventional humic substances.
Strengths: Highly specialized product formulations for specific metal contamination scenarios; enhanced stability across varying environmental conditions; commercial-scale production capabilities. Weaknesses: Their proprietary enhancement process increases production costs, making solutions less economical for large-scale remediation projects compared to conventional humic substances.
Technical Mechanisms of Metal-Organic Complexation
Method for coloring various rock types
PatentInactiveUS20050163936A1
Innovation
- Utilizing Humic acid or Fulvic acid, environmentally safe organic acids with high cation exchange capacity, to chelate transition metal salts at low molar concentrations, reducing the need for high metal salt usage and avoiding harmful acids like Acetic or Hydrochloric Acid, thereby creating a safer, more environmentally friendly method for simulating desert varnish on rock surfaces.
Novel Method to Formulate Humic Substances
PatentInactiveUS20230078902A1
Innovation
- A blockchain-based multichain protocol is introduced to track the lifecycle of humic substances from mining to application in agriculture, providing a secure and transparent system for issuing and retiring carbon credits, ensuring the authenticity of emissions reductions and incentivizing farmers to adopt humic substance-based fertilizers over synthetic nitrogen.
Environmental Impact Assessment of Acid-Based Remediation
The environmental implications of using fulvic and humic acids for toxic metal remediation extend beyond their immediate effectiveness in absorption. These organic compounds, when deployed in contaminated environments, interact with complex ecosystems in ways that require comprehensive assessment.
Fulvic acid-based remediation typically demonstrates lower environmental persistence compared to humic acid treatments. When introduced into soil or water systems, fulvic acids degrade more rapidly, reducing long-term ecological alterations. This characteristic makes fulvic acid potentially preferable in sensitive ecosystems where minimal intervention footprints are desired.
The pH modification resulting from acid-based remediation presents significant ecological considerations. Humic acids generally cause more substantial pH shifts in treated environments, potentially disrupting microbial communities and altering nutrient availability for plants. Studies indicate that soil enzyme activities, particularly those involved in carbon and nitrogen cycling, show variable responses to humic acid applications, with potential inhibition at higher concentrations.
Water quality impacts differ markedly between these remediation approaches. Fulvic acid treatments typically result in less coloration and turbidity increases in aquatic environments, maintaining better light penetration essential for photosynthetic organisms. Conversely, humic acid applications often produce darker water coloration that may temporarily reduce photosynthesis rates in affected water bodies.
Secondary metabolite production in both acid types warrants careful monitoring. These compounds can influence microbial community structures, potentially favoring certain species over others. Research indicates that humic acids tend to stimulate greater shifts in microbial population dynamics, which may either enhance or disrupt existing ecological functions depending on site-specific conditions.
Bioaccumulation potential represents another critical environmental consideration. While both acids effectively bind toxic metals, the stability of these complexes under varying environmental conditions determines whether metals might be remobilized over time. Fulvic acid-metal complexes generally demonstrate higher solubility, potentially increasing mobility through environmental compartments, whereas humic acid typically forms more stable, less mobile complexes.
Ecosystem recovery trajectories following acid-based remediation show that fulvic acid treated sites often return to baseline conditions more rapidly. This accelerated recovery may be attributed to the lower molecular weight and higher reactivity of fulvic acids, allowing faster integration into natural carbon cycles without persistent ecosystem alterations.
Fulvic acid-based remediation typically demonstrates lower environmental persistence compared to humic acid treatments. When introduced into soil or water systems, fulvic acids degrade more rapidly, reducing long-term ecological alterations. This characteristic makes fulvic acid potentially preferable in sensitive ecosystems where minimal intervention footprints are desired.
The pH modification resulting from acid-based remediation presents significant ecological considerations. Humic acids generally cause more substantial pH shifts in treated environments, potentially disrupting microbial communities and altering nutrient availability for plants. Studies indicate that soil enzyme activities, particularly those involved in carbon and nitrogen cycling, show variable responses to humic acid applications, with potential inhibition at higher concentrations.
Water quality impacts differ markedly between these remediation approaches. Fulvic acid treatments typically result in less coloration and turbidity increases in aquatic environments, maintaining better light penetration essential for photosynthetic organisms. Conversely, humic acid applications often produce darker water coloration that may temporarily reduce photosynthesis rates in affected water bodies.
Secondary metabolite production in both acid types warrants careful monitoring. These compounds can influence microbial community structures, potentially favoring certain species over others. Research indicates that humic acids tend to stimulate greater shifts in microbial population dynamics, which may either enhance or disrupt existing ecological functions depending on site-specific conditions.
Bioaccumulation potential represents another critical environmental consideration. While both acids effectively bind toxic metals, the stability of these complexes under varying environmental conditions determines whether metals might be remobilized over time. Fulvic acid-metal complexes generally demonstrate higher solubility, potentially increasing mobility through environmental compartments, whereas humic acid typically forms more stable, less mobile complexes.
Ecosystem recovery trajectories following acid-based remediation show that fulvic acid treated sites often return to baseline conditions more rapidly. This accelerated recovery may be attributed to the lower molecular weight and higher reactivity of fulvic acids, allowing faster integration into natural carbon cycles without persistent ecosystem alterations.
Regulatory Framework for Soil and Water Treatment Technologies
The regulatory landscape governing soil and water treatment technologies, particularly those involving fulvic and humic acids for toxic metal remediation, is complex and multifaceted across different jurisdictions. In the United States, the Environmental Protection Agency (EPA) regulates these substances under multiple frameworks including the Safe Drinking Water Act (SDWA), the Clean Water Act (CWA), and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). The EPA has established specific guidelines for permissible levels of heavy metals in soil and water, which directly impacts the application of humic substances in remediation efforts.
The European Union operates under the Water Framework Directive (2000/60/EC) and the Groundwater Directive (2006/118/EC), which set comprehensive standards for water quality and pollution control. The European Chemicals Agency (ECHA) additionally regulates humic and fulvic acid products under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, requiring extensive safety data before market approval.
In Asia, regulatory frameworks vary significantly by country. Japan's Ministry of the Environment enforces the Soil Contamination Countermeasures Act, while China has implemented the Soil Pollution Prevention and Control Law in 2019, representing a significant advancement in their environmental regulatory system. These frameworks increasingly recognize natural remediation technologies, including humic substance applications.
International standards organizations, particularly ISO, have developed specific protocols for testing the effectiveness of remediation technologies. ISO 18772:2008 provides guidelines for leaching procedures for environmental risk assessment, which is particularly relevant when evaluating humic and fulvic acid applications for metal absorption.
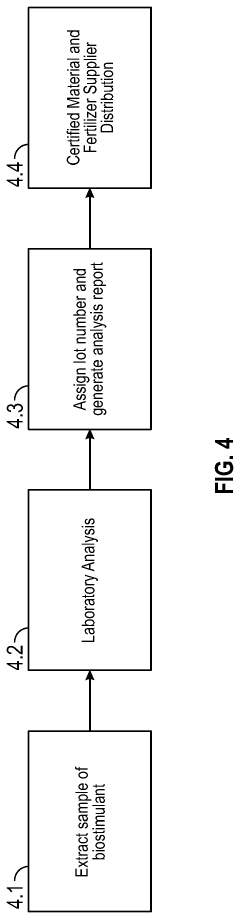
Certification requirements for commercial humic and fulvic acid products vary widely. In agricultural applications, many countries require registration similar to fertilizers or soil amendments, while environmental remediation applications often require demonstration of efficacy and environmental safety through standardized testing protocols.
Recent regulatory trends show increasing acceptance of nature-based solutions for environmental remediation. Several jurisdictions have introduced fast-track approval processes for technologies demonstrating lower environmental impact compared to traditional chemical treatments. This regulatory evolution has created more favorable conditions for humic substance-based remediation technologies.
Compliance challenges remain significant, particularly regarding consistent quality control of naturally derived humic substances and standardization of testing methodologies to verify metal absorption efficacy. The variable composition of these natural products presents unique regulatory challenges that conventional chemical treatments do not face.
The European Union operates under the Water Framework Directive (2000/60/EC) and the Groundwater Directive (2006/118/EC), which set comprehensive standards for water quality and pollution control. The European Chemicals Agency (ECHA) additionally regulates humic and fulvic acid products under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, requiring extensive safety data before market approval.
In Asia, regulatory frameworks vary significantly by country. Japan's Ministry of the Environment enforces the Soil Contamination Countermeasures Act, while China has implemented the Soil Pollution Prevention and Control Law in 2019, representing a significant advancement in their environmental regulatory system. These frameworks increasingly recognize natural remediation technologies, including humic substance applications.
International standards organizations, particularly ISO, have developed specific protocols for testing the effectiveness of remediation technologies. ISO 18772:2008 provides guidelines for leaching procedures for environmental risk assessment, which is particularly relevant when evaluating humic and fulvic acid applications for metal absorption.
Certification requirements for commercial humic and fulvic acid products vary widely. In agricultural applications, many countries require registration similar to fertilizers or soil amendments, while environmental remediation applications often require demonstration of efficacy and environmental safety through standardized testing protocols.
Recent regulatory trends show increasing acceptance of nature-based solutions for environmental remediation. Several jurisdictions have introduced fast-track approval processes for technologies demonstrating lower environmental impact compared to traditional chemical treatments. This regulatory evolution has created more favorable conditions for humic substance-based remediation technologies.
Compliance challenges remain significant, particularly regarding consistent quality control of naturally derived humic substances and standardization of testing methodologies to verify metal absorption efficacy. The variable composition of these natural products presents unique regulatory challenges that conventional chemical treatments do not face.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!