Evaluation of biomimetic scaffolds for drug delivery applications
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomimetic Scaffolds Background and Objectives
Biomimetic scaffolds represent a revolutionary approach in drug delivery systems, mimicking natural tissue structures to enhance therapeutic efficacy while minimizing side effects. The evolution of these scaffolds traces back to the early 2000s when researchers began exploring biomimetic principles in material design. Initially focused on simple structural mimicry, the field has progressively incorporated more sophisticated biological functions, including cell-material interactions, enzyme-responsive elements, and tissue-specific targeting mechanisms.
The technological trajectory has been shaped by advances in materials science, nanotechnology, and molecular biology, converging to create increasingly complex and functional delivery platforms. Recent developments have particularly emphasized stimuli-responsive scaffolds that can release therapeutic agents in response to specific physiological or pathological conditions, representing a significant leap toward precision medicine.
Current research objectives in biomimetic scaffold development center on addressing several critical challenges. Primary among these is achieving precise spatial and temporal control over drug release kinetics to match therapeutic requirements. Additionally, researchers aim to enhance biocompatibility and biodegradability profiles while maintaining structural integrity during the required therapeutic window.
Another key objective involves improving the scalability and reproducibility of manufacturing processes to facilitate clinical translation. Despite promising laboratory results, many biomimetic scaffold technologies face significant hurdles in scaling production while maintaining consistent quality and performance characteristics.
The integration of multiple functionalities within a single scaffold system represents another frontier in this field. Modern therapeutic approaches often require combination treatments, necessitating scaffolds capable of delivering multiple bioactive agents with distinct release profiles. This multi-modal approach aims to address complex pathological conditions through synergistic therapeutic effects.
Looking forward, the field is moving toward personalized biomimetic scaffolds tailored to individual patient needs. This patient-specific approach considers factors such as genetic makeup, disease state, and physiological parameters to optimize therapeutic outcomes. Advanced manufacturing technologies, particularly 3D bioprinting and microfluidic fabrication, are enabling this transition toward personalized scaffold-based drug delivery systems.
The ultimate goal remains the development of "smart" biomimetic scaffolds that can autonomously respond to the dynamic physiological environment, adjusting drug release parameters in real-time based on therapeutic needs. This vision represents the convergence of biomaterials science with emerging technologies in sensors, artificial intelligence, and responsive materials.
The technological trajectory has been shaped by advances in materials science, nanotechnology, and molecular biology, converging to create increasingly complex and functional delivery platforms. Recent developments have particularly emphasized stimuli-responsive scaffolds that can release therapeutic agents in response to specific physiological or pathological conditions, representing a significant leap toward precision medicine.
Current research objectives in biomimetic scaffold development center on addressing several critical challenges. Primary among these is achieving precise spatial and temporal control over drug release kinetics to match therapeutic requirements. Additionally, researchers aim to enhance biocompatibility and biodegradability profiles while maintaining structural integrity during the required therapeutic window.
Another key objective involves improving the scalability and reproducibility of manufacturing processes to facilitate clinical translation. Despite promising laboratory results, many biomimetic scaffold technologies face significant hurdles in scaling production while maintaining consistent quality and performance characteristics.
The integration of multiple functionalities within a single scaffold system represents another frontier in this field. Modern therapeutic approaches often require combination treatments, necessitating scaffolds capable of delivering multiple bioactive agents with distinct release profiles. This multi-modal approach aims to address complex pathological conditions through synergistic therapeutic effects.
Looking forward, the field is moving toward personalized biomimetic scaffolds tailored to individual patient needs. This patient-specific approach considers factors such as genetic makeup, disease state, and physiological parameters to optimize therapeutic outcomes. Advanced manufacturing technologies, particularly 3D bioprinting and microfluidic fabrication, are enabling this transition toward personalized scaffold-based drug delivery systems.
The ultimate goal remains the development of "smart" biomimetic scaffolds that can autonomously respond to the dynamic physiological environment, adjusting drug release parameters in real-time based on therapeutic needs. This vision represents the convergence of biomaterials science with emerging technologies in sensors, artificial intelligence, and responsive materials.
Market Analysis for Drug Delivery Scaffold Systems
The global market for drug delivery scaffold systems has experienced significant growth in recent years, driven by increasing prevalence of chronic diseases and rising demand for targeted drug delivery solutions. Currently valued at approximately 5.8 billion USD in 2023, this market segment is projected to reach 9.7 billion USD by 2028, representing a compound annual growth rate (CAGR) of 10.8% during the forecast period.
Biomimetic scaffolds represent a rapidly expanding subsector within this market, accounting for roughly 1.2 billion USD in 2023, with projections indicating growth to 2.5 billion USD by 2028. This accelerated growth rate of 15.9% CAGR reflects the increasing recognition of biomimetic approaches in enhancing therapeutic outcomes.
Geographically, North America dominates the market with approximately 42% share, followed by Europe (28%), Asia-Pacific (21%), and rest of the world (9%). However, the Asia-Pacific region is experiencing the fastest growth rate at 17.3% annually, primarily driven by increasing healthcare expenditure in China and India, along with expanding research infrastructure.
By application segment, cancer therapy represents the largest market share (38%), followed by tissue regeneration (27%), wound healing (18%), and other applications (17%). The oncology segment's dominance stems from the critical need for targeted delivery systems that minimize systemic toxicity while maximizing therapeutic efficacy at tumor sites.
Key market drivers include increasing prevalence of chronic diseases, growing demand for minimally invasive drug delivery methods, rising healthcare expenditure, and technological advancements in biomaterials science. Additionally, the shift toward personalized medicine is creating new opportunities for customized scaffold systems tailored to individual patient needs.
Market restraints include high development costs, stringent regulatory requirements, and technical challenges in achieving controlled release profiles. The average development timeline for a new scaffold system spans 4-6 years, with regulatory approval processes adding another 1-3 years before commercialization.
Customer segmentation reveals pharmaceutical companies as primary purchasers (53%), followed by research institutions (27%), hospitals and clinics (15%), and others (5%). End-user preferences increasingly favor biodegradable systems with tunable release profiles and targeting capabilities.
Future market trends indicate growing integration of smart technologies, including stimuli-responsive scaffolds and digital monitoring capabilities. Additionally, combination products incorporating multiple therapeutic agents within single scaffold systems are gaining traction, offering potential for addressing complex disease pathologies through synchronized delivery mechanisms.
Biomimetic scaffolds represent a rapidly expanding subsector within this market, accounting for roughly 1.2 billion USD in 2023, with projections indicating growth to 2.5 billion USD by 2028. This accelerated growth rate of 15.9% CAGR reflects the increasing recognition of biomimetic approaches in enhancing therapeutic outcomes.
Geographically, North America dominates the market with approximately 42% share, followed by Europe (28%), Asia-Pacific (21%), and rest of the world (9%). However, the Asia-Pacific region is experiencing the fastest growth rate at 17.3% annually, primarily driven by increasing healthcare expenditure in China and India, along with expanding research infrastructure.
By application segment, cancer therapy represents the largest market share (38%), followed by tissue regeneration (27%), wound healing (18%), and other applications (17%). The oncology segment's dominance stems from the critical need for targeted delivery systems that minimize systemic toxicity while maximizing therapeutic efficacy at tumor sites.
Key market drivers include increasing prevalence of chronic diseases, growing demand for minimally invasive drug delivery methods, rising healthcare expenditure, and technological advancements in biomaterials science. Additionally, the shift toward personalized medicine is creating new opportunities for customized scaffold systems tailored to individual patient needs.
Market restraints include high development costs, stringent regulatory requirements, and technical challenges in achieving controlled release profiles. The average development timeline for a new scaffold system spans 4-6 years, with regulatory approval processes adding another 1-3 years before commercialization.
Customer segmentation reveals pharmaceutical companies as primary purchasers (53%), followed by research institutions (27%), hospitals and clinics (15%), and others (5%). End-user preferences increasingly favor biodegradable systems with tunable release profiles and targeting capabilities.
Future market trends indicate growing integration of smart technologies, including stimuli-responsive scaffolds and digital monitoring capabilities. Additionally, combination products incorporating multiple therapeutic agents within single scaffold systems are gaining traction, offering potential for addressing complex disease pathologies through synchronized delivery mechanisms.
Current Challenges in Biomimetic Drug Delivery
Despite significant advancements in biomimetic scaffold technology for drug delivery applications, several critical challenges continue to impede widespread clinical implementation. One of the foremost obstacles remains the precise control of drug release kinetics. Current biomimetic scaffolds often exhibit burst release profiles, where a substantial portion of the therapeutic payload is discharged rapidly following administration, followed by suboptimal sustained release. This phenomenon significantly limits therapeutic efficacy and potentially increases systemic toxicity.
Material biocompatibility presents another substantial hurdle. While biomimetic approaches aim to replicate natural tissue environments, achieving complete immunological acceptance remains elusive. Many scaffolds trigger foreign body responses, leading to inflammation, fibrous encapsulation, or premature degradation, which compromises their drug delivery functionality and patient safety profiles.
Scale-up manufacturing represents a significant technical barrier. Laboratory-scale production of biomimetic scaffolds often employs sophisticated techniques that prove challenging to translate to industrial-scale manufacturing while maintaining consistent quality attributes. This manufacturing gap creates substantial obstacles for commercial viability and regulatory approval pathways.
Stability concerns during storage and after administration constitute another critical challenge. Many biomimetic materials undergo structural alterations or degradation under physiological conditions at rates that do not align optimally with therapeutic requirements. Controlling degradation kinetics to match drug release profiles remains technically demanding.
Targeting specificity presents ongoing difficulties. Despite biomimetic design principles, achieving precise localization at intended therapeutic sites while minimizing off-target effects continues to challenge researchers. The complex biological milieu often interferes with targeting mechanisms, reducing delivery efficiency.
Regulatory hurdles compound these technical challenges. The novel and complex nature of biomimetic scaffolds creates uncertainty in regulatory classification and approval pathways. Demonstrating safety, efficacy, and manufacturing consistency to regulatory authorities requires extensive validation studies that many academic and early-stage commercial entities struggle to complete.
Cost-effectiveness remains a significant barrier to widespread adoption. Current production methods for sophisticated biomimetic scaffolds often involve expensive materials, complex fabrication techniques, and extensive quality control measures, resulting in prohibitive costs that limit commercial viability and patient accessibility.
Material biocompatibility presents another substantial hurdle. While biomimetic approaches aim to replicate natural tissue environments, achieving complete immunological acceptance remains elusive. Many scaffolds trigger foreign body responses, leading to inflammation, fibrous encapsulation, or premature degradation, which compromises their drug delivery functionality and patient safety profiles.
Scale-up manufacturing represents a significant technical barrier. Laboratory-scale production of biomimetic scaffolds often employs sophisticated techniques that prove challenging to translate to industrial-scale manufacturing while maintaining consistent quality attributes. This manufacturing gap creates substantial obstacles for commercial viability and regulatory approval pathways.
Stability concerns during storage and after administration constitute another critical challenge. Many biomimetic materials undergo structural alterations or degradation under physiological conditions at rates that do not align optimally with therapeutic requirements. Controlling degradation kinetics to match drug release profiles remains technically demanding.
Targeting specificity presents ongoing difficulties. Despite biomimetic design principles, achieving precise localization at intended therapeutic sites while minimizing off-target effects continues to challenge researchers. The complex biological milieu often interferes with targeting mechanisms, reducing delivery efficiency.
Regulatory hurdles compound these technical challenges. The novel and complex nature of biomimetic scaffolds creates uncertainty in regulatory classification and approval pathways. Demonstrating safety, efficacy, and manufacturing consistency to regulatory authorities requires extensive validation studies that many academic and early-stage commercial entities struggle to complete.
Cost-effectiveness remains a significant barrier to widespread adoption. Current production methods for sophisticated biomimetic scaffolds often involve expensive materials, complex fabrication techniques, and extensive quality control measures, resulting in prohibitive costs that limit commercial viability and patient accessibility.
Current Biomimetic Scaffold Design Approaches
01 Natural polymer-based biomimetic scaffolds
Biomimetic scaffolds can be created using natural polymers such as collagen, silk, and cellulose to mimic the extracellular matrix of native tissues. These natural materials provide biocompatibility and biodegradability while supporting cell adhesion, proliferation, and differentiation. The scaffolds can be processed into various forms including hydrogels, fibers, and porous structures to match the mechanical and biological properties of target tissues.- Natural polymer-based biomimetic scaffolds: Biomimetic scaffolds can be created using natural polymers such as collagen, silk, and cellulose to mimic the extracellular matrix of native tissues. These natural materials provide biocompatibility and biodegradability while supporting cell adhesion, proliferation, and differentiation. The scaffolds can be processed into various forms including hydrogels, fibers, and porous structures to match the mechanical and biological properties of target tissues.
- 3D printing of biomimetic scaffolds: Advanced 3D printing technologies enable the fabrication of complex biomimetic scaffolds with precise control over architecture, porosity, and mechanical properties. These techniques allow for patient-specific designs that can incorporate multiple materials and bioactive components. 3D printed scaffolds can be customized to match the anatomical features of target tissues and include gradients of properties to better mimic native tissue organization.
- Biomimetic scaffolds for tissue engineering and regeneration: Scaffolds designed to mimic the native tissue environment can promote tissue regeneration by providing structural support and biochemical cues. These scaffolds incorporate features such as controlled degradation rates, appropriate mechanical properties, and bioactive molecules to guide cell behavior. Applications include bone, cartilage, skin, neural, and cardiovascular tissue engineering, where the scaffolds serve as temporary templates for new tissue formation.
- Surface modification of biomimetic scaffolds: The surface properties of biomimetic scaffolds can be modified to enhance cell attachment, proliferation, and differentiation. Techniques include coating with bioactive molecules, plasma treatment, and chemical functionalization to create specific binding sites for cells. These modifications can introduce biomimetic features such as nanoscale topography, controlled hydrophilicity, and presentation of cell adhesion motifs to better mimic the natural cellular microenvironment.
- Composite and hybrid biomimetic scaffolds: Composite and hybrid biomimetic scaffolds combine multiple materials to achieve enhanced properties that better mimic native tissues. These scaffolds may incorporate combinations of natural and synthetic polymers, ceramics, or metals to optimize mechanical strength, degradation profiles, and biological responses. The integration of nanoparticles, growth factors, and other bioactive components can further enhance the scaffolds' ability to guide tissue formation and vascularization.
02 3D printing techniques for biomimetic scaffold fabrication
Advanced 3D printing technologies enable the fabrication of complex biomimetic scaffolds with precise control over architecture, porosity, and mechanical properties. These techniques allow for patient-specific designs and the incorporation of multiple materials and bioactive components. 3D bioprinting can create hierarchical structures that mimic native tissue organization, with controlled gradients of properties and integrated vascular networks to support cell viability in larger constructs.Expand Specific Solutions03 Tissue-specific biomimetic scaffolds for regenerative medicine
Specialized biomimetic scaffolds can be engineered to replicate the specific microenvironments of different tissues such as bone, cartilage, skin, and neural tissue. These scaffolds incorporate tissue-specific biochemical cues, mechanical properties, and architectural features to guide appropriate cell behavior and tissue formation. By mimicking the native tissue environment, these scaffolds promote more effective tissue regeneration and functional recovery in various clinical applications.Expand Specific Solutions04 Surface modification of biomimetic scaffolds
The surface properties of biomimetic scaffolds can be modified to enhance cell attachment, proliferation, and differentiation. Techniques include coating with bioactive molecules, peptides, growth factors, or extracellular matrix components. Surface topography can be engineered at micro and nanoscales to influence cell behavior. These modifications improve the biological performance of scaffolds by providing specific recognition sites for cells and promoting desired cellular responses.Expand Specific Solutions05 Composite and hybrid biomimetic scaffolds
Composite and hybrid biomimetic scaffolds combine multiple materials to achieve superior properties that cannot be obtained with single materials. These scaffolds often integrate natural and synthetic polymers, ceramics, or metals to optimize mechanical strength, degradation rates, and biological activity. The synergistic combination of different materials creates scaffolds that better mimic the complex composition and hierarchical structure of natural tissues, leading to improved functional outcomes in tissue engineering applications.Expand Specific Solutions
Leading Organizations in Biomimetic Drug Delivery
The biomimetic scaffold drug delivery market is currently in a growth phase, characterized by increasing research activities and commercial applications. The global market size is estimated to reach $5-7 billion by 2027, driven by rising demand for targeted drug delivery systems. Technologically, the field is advancing rapidly but remains in mid-maturity, with significant innovation potential. Key players demonstrate varying levels of technological sophistication: Mersana Therapeutics and Regeneron Pharmaceuticals lead with advanced antibody-drug conjugate platforms, while Sanofi and W.L. Gore & Associates contribute significant R&D resources. Academic institutions like Boston University, Northwestern University, and UNC Chapel Hill provide crucial research foundations. Medtronic Vascular and Abbott Cardiovascular Systems are leveraging their medical device expertise to develop innovative scaffold-based delivery systems, creating a competitive landscape balanced between pharmaceutical giants and specialized biotech innovators.
Mersana Therapeutics, Inc.
Technical Solution: Mersana has developed the Fleximer® platform, a biodegradable, biocompatible, and highly versatile polymer scaffold system specifically designed for drug delivery applications. This technology enables the conjugation of multiple therapeutic payloads to a single polymer backbone, creating what they term Antibody-Drug Conjugates (ADCs). Their Dolaflexin platform incorporates their proprietary auristatin payload with a controlled biolabile linker system that allows for precise drug release in tumor microenvironments. The biomimetic nature of their scaffolds lies in their ability to mimic natural biological processes, with the polymer backbone designed to navigate biological barriers while maintaining stability in circulation and facilitating controlled release at target sites. Their XMT-1536 candidate, utilizing this scaffold technology, has demonstrated significant efficacy in ovarian cancer models with improved therapeutic index compared to conventional delivery methods.
Strengths: Highly customizable platform allowing precise payload-to-antibody ratios; reduced off-target toxicity through controlled release mechanisms; improved drug solubility and pharmacokinetics. Weaknesses: Complex manufacturing process; potential immunogenicity concerns with repeated administration; higher production costs compared to conventional drug formulations.
President & Fellows of Harvard College
Technical Solution: Harvard's research teams have pioneered several biomimetic scaffold technologies for drug delivery, with particular emphasis on organ-on-chip platforms and shear-responsive biomaterials. Their notable innovation includes the development of DNA-origami based nanostructures that mimic the architecture and functionality of biological systems. These programmable scaffolds can be precisely engineered at the nanoscale to create 3D structures that respond to specific biological triggers. Harvard researchers have also developed injectable hydrogels with extracellular matrix-mimicking properties that provide sustained release of therapeutics while supporting tissue regeneration. Their biomimetic vascular network technology enables the creation of perfusable blood vessel networks within scaffolds, allowing for more physiologically relevant drug testing and delivery systems. Additionally, their shape-memory scaffold systems can change configuration in response to temperature or pH, enabling targeted delivery to specific anatomical locations with minimal invasiveness during implantation.
Strengths: Exceptional precision in scaffold architecture control; highly sophisticated biomimetic properties that closely replicate natural tissue environments; strong integration of multiple technologies (microfluidics, materials science, molecular biology). Weaknesses: Many technologies remain in pre-clinical stages; complex fabrication processes may limit scalability; higher production costs compared to conventional drug delivery systems.
Key Patents in Biomimetic Drug Delivery Systems
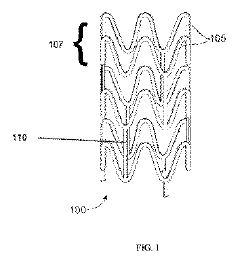
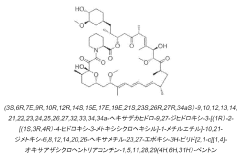
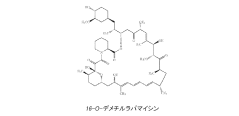
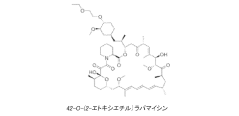
Drug delivery scaffold or stent with a novolimus and lactide based coating such that novolimus has a minimum amount of bonding to the coating
PatentPendingHK1216509A
Innovation
- A drug delivery system where a macrocyclic drug like novolimus is conjugated to a poly(D,L-lactide) polymer coating with a minimal amount of less than 0.35% weight novolimus to weight polymer, as measured by LC-MS, to minimize drug dose reduction and avoid generating unknown biological and toxicological species.
A drug delivery scaffold or stent with a coating based on novolimus and lactide such that binding of novolimus to the coating is minimized
PatentInactiveJP2017505817A
Innovation
- The use of a poly(D,L-lactide) polymer with novolimus conjugated at a maximum of 0.35% by weight, minimizing drug-polymer reactions and avoiding the formation of new species, achieved through methods like liquid chromatography-mass spectrometry for precise drug-polymer conjugate control.
Biocompatibility and Safety Considerations
Biocompatibility remains a paramount concern in the development of biomimetic scaffolds for drug delivery applications. These scaffolds must interact harmoniously with biological systems without eliciting adverse immune responses or toxicity. The materials selected for scaffold fabrication directly influence biocompatibility outcomes, with natural polymers like collagen, chitosan, and alginate generally demonstrating superior biocompatibility compared to synthetic alternatives. However, even natural materials require thorough evaluation to ensure they do not trigger inflammatory cascades or immune rejection when introduced into specific physiological environments.
Safety considerations extend beyond immediate biocompatibility to encompass long-term effects, including degradation profiles and metabolite toxicity. Biomimetic scaffolds designed for controlled drug release must degrade at predictable rates that align with therapeutic objectives while producing non-toxic byproducts that can be effectively cleared from the body. Recent studies have demonstrated that scaffold architecture significantly impacts safety profiles, with certain pore sizes and distributions potentially causing localized inflammation or impeding normal tissue function.
Regulatory frameworks worldwide have established stringent protocols for evaluating the safety of biomimetic drug delivery systems. These typically include in vitro cytotoxicity assessments, genotoxicity studies, sensitization tests, and in vivo biocompatibility evaluations. The ISO 10993 standards series provides comprehensive guidelines for biological evaluation of medical devices, including drug-eluting scaffolds. Compliance with these standards represents a critical milestone in translating biomimetic scaffold technologies from laboratory research to clinical applications.
Advanced imaging techniques have revolutionized safety monitoring capabilities, allowing researchers to track scaffold degradation and tissue integration in real-time. Techniques such as intravital microscopy and non-invasive imaging modalities provide valuable insights into host-scaffold interactions that were previously unattainable. These methodologies have revealed that surface modifications, such as protein coatings or specific ligand attachments, can significantly enhance biocompatibility while maintaining drug delivery efficacy.
Emerging research focuses on developing "stealth" biomimetic scaffolds that evade immune surveillance through strategic surface engineering. By mimicking cell membrane components or incorporating immunomodulatory molecules, these advanced scaffolds aim to minimize foreign body responses while maximizing therapeutic payload delivery. Additionally, personalized approaches utilizing patient-derived materials show promise in reducing immunogenicity concerns, though they present challenges related to standardization and scalability in manufacturing processes.
Safety considerations extend beyond immediate biocompatibility to encompass long-term effects, including degradation profiles and metabolite toxicity. Biomimetic scaffolds designed for controlled drug release must degrade at predictable rates that align with therapeutic objectives while producing non-toxic byproducts that can be effectively cleared from the body. Recent studies have demonstrated that scaffold architecture significantly impacts safety profiles, with certain pore sizes and distributions potentially causing localized inflammation or impeding normal tissue function.
Regulatory frameworks worldwide have established stringent protocols for evaluating the safety of biomimetic drug delivery systems. These typically include in vitro cytotoxicity assessments, genotoxicity studies, sensitization tests, and in vivo biocompatibility evaluations. The ISO 10993 standards series provides comprehensive guidelines for biological evaluation of medical devices, including drug-eluting scaffolds. Compliance with these standards represents a critical milestone in translating biomimetic scaffold technologies from laboratory research to clinical applications.
Advanced imaging techniques have revolutionized safety monitoring capabilities, allowing researchers to track scaffold degradation and tissue integration in real-time. Techniques such as intravital microscopy and non-invasive imaging modalities provide valuable insights into host-scaffold interactions that were previously unattainable. These methodologies have revealed that surface modifications, such as protein coatings or specific ligand attachments, can significantly enhance biocompatibility while maintaining drug delivery efficacy.
Emerging research focuses on developing "stealth" biomimetic scaffolds that evade immune surveillance through strategic surface engineering. By mimicking cell membrane components or incorporating immunomodulatory molecules, these advanced scaffolds aim to minimize foreign body responses while maximizing therapeutic payload delivery. Additionally, personalized approaches utilizing patient-derived materials show promise in reducing immunogenicity concerns, though they present challenges related to standardization and scalability in manufacturing processes.
Regulatory Pathway for Biomimetic Drug Delivery Systems
The regulatory landscape for biomimetic drug delivery systems presents a complex pathway that developers must navigate to bring innovative products to market. In the United States, the FDA typically classifies these systems as combination products, requiring review by multiple centers including the Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), and Center for Devices and Radiological Health (CDRH). The primary mode of action determines which center takes the lead in the review process.
For biomimetic scaffolds specifically, developers must first establish the classification of their product through pre-submission meetings with the FDA. These scaffolds often fall under the combination product category due to their drug delivery function coupled with structural biomimetic properties. The regulatory pathway generally includes preclinical testing, Investigational New Drug (IND) application, clinical trials phases I-III, and New Drug Application (NDA) or Biologics License Application (BLA) submission.
European regulatory frameworks, governed by the European Medicines Agency (EMA), require CE marking for medical devices and additional approval processes for drug components. The classification of biomimetic scaffolds in Europe often falls under Class III medical devices when they incorporate medicinal substances, necessitating consultation with medicinal product authorities.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have established guidelines that facilitate global development of these technologies. However, significant regional differences remain in regulatory approaches, particularly regarding the classification of biomimetic materials.
Quality control standards present another critical regulatory consideration. Manufacturers must implement Good Manufacturing Practices (GMP) and establish robust quality management systems that address the unique challenges of biomimetic scaffolds, including material consistency, sterility, and stability of incorporated bioactive compounds.
Post-market surveillance requirements are particularly stringent for biomimetic drug delivery systems due to their novel nature and potential long-term effects. Manufacturers must maintain vigilance systems to monitor adverse events and implement risk management plans that extend throughout the product lifecycle.
Regulatory trends indicate movement toward adaptive licensing pathways for innovative technologies like biomimetic scaffolds, potentially accelerating market access while maintaining rigorous safety standards. Early engagement with regulatory authorities through programs like the FDA's Breakthrough Devices Program or the EMA's PRIME (Priority Medicines) scheme can provide strategic advantages for developers navigating this complex regulatory landscape.
For biomimetic scaffolds specifically, developers must first establish the classification of their product through pre-submission meetings with the FDA. These scaffolds often fall under the combination product category due to their drug delivery function coupled with structural biomimetic properties. The regulatory pathway generally includes preclinical testing, Investigational New Drug (IND) application, clinical trials phases I-III, and New Drug Application (NDA) or Biologics License Application (BLA) submission.
European regulatory frameworks, governed by the European Medicines Agency (EMA), require CE marking for medical devices and additional approval processes for drug components. The classification of biomimetic scaffolds in Europe often falls under Class III medical devices when they incorporate medicinal substances, necessitating consultation with medicinal product authorities.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have established guidelines that facilitate global development of these technologies. However, significant regional differences remain in regulatory approaches, particularly regarding the classification of biomimetic materials.
Quality control standards present another critical regulatory consideration. Manufacturers must implement Good Manufacturing Practices (GMP) and establish robust quality management systems that address the unique challenges of biomimetic scaffolds, including material consistency, sterility, and stability of incorporated bioactive compounds.
Post-market surveillance requirements are particularly stringent for biomimetic drug delivery systems due to their novel nature and potential long-term effects. Manufacturers must maintain vigilance systems to monitor adverse events and implement risk management plans that extend throughout the product lifecycle.
Regulatory trends indicate movement toward adaptive licensing pathways for innovative technologies like biomimetic scaffolds, potentially accelerating market access while maintaining rigorous safety standards. Early engagement with regulatory authorities through programs like the FDA's Breakthrough Devices Program or the EMA's PRIME (Priority Medicines) scheme can provide strategic advantages for developers navigating this complex regulatory landscape.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!