Research on biomimetic scaffolds with nanofiber architectures
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomimetic Nanofiber Scaffolds: Background and Objectives
Biomimetic scaffolds with nanofiber architectures represent a revolutionary approach in tissue engineering and regenerative medicine, drawing inspiration from nature's intricate designs. The evolution of this technology can be traced back to the early 2000s when electrospinning techniques were first applied to create nanofibrous structures for biomedical applications. Since then, the field has witnessed exponential growth, driven by advances in materials science, nanotechnology, and biological understanding of cell-matrix interactions.
The fundamental principle behind biomimetic nanofiber scaffolds lies in their ability to mimic the native extracellular matrix (ECM) at the nanoscale level. Natural ECM consists of a complex network of protein fibers with diameters ranging from 50-500 nm, providing both structural support and biochemical cues to cells. By replicating these architectural features, researchers aim to create more physiologically relevant environments for cell growth, differentiation, and tissue formation.
Recent technological trends in this field include the development of multi-component nanofibers, stimuli-responsive scaffolds, and hierarchically structured materials that better recapitulate the complexity of native tissues. The integration of bioactive molecules, growth factors, and cell-instructive signals within nanofiber architectures has further enhanced their biomimetic properties, enabling more precise control over cellular behavior and tissue development.
The global research landscape shows a clear trajectory toward more sophisticated fabrication techniques beyond conventional electrospinning, including rotary jet spinning, solution blow spinning, and 3D bioprinting of nanofibers. These advances have expanded the potential applications of nanofiber scaffolds from simple tissue substitutes to complex organ-on-chip models and drug delivery systems.
The primary objectives of current research in biomimetic nanofiber scaffolds encompass several dimensions. First, improving the mechanical properties to match those of target tissues while maintaining appropriate porosity and degradation profiles. Second, enhancing biocompatibility and bioactivity through surface functionalization and incorporation of cell-signaling molecules. Third, developing scalable manufacturing processes that can translate laboratory successes into clinically viable products.
Another critical goal is to achieve precise spatial control over fiber orientation, alignment, and density to recreate the anisotropic nature of many native tissues. This directional organization is particularly important for tissues like muscle, tendon, and neural structures where cellular alignment directly influences functionality.
Looking forward, the field aims to develop "smart" nanofiber scaffolds capable of responding dynamically to cellular activities and environmental cues, potentially revolutionizing personalized medicine approaches. The ultimate technical objective remains the creation of fully functional, transplantable tissue constructs that can seamlessly integrate with the host and restore normal physiological function.
The fundamental principle behind biomimetic nanofiber scaffolds lies in their ability to mimic the native extracellular matrix (ECM) at the nanoscale level. Natural ECM consists of a complex network of protein fibers with diameters ranging from 50-500 nm, providing both structural support and biochemical cues to cells. By replicating these architectural features, researchers aim to create more physiologically relevant environments for cell growth, differentiation, and tissue formation.
Recent technological trends in this field include the development of multi-component nanofibers, stimuli-responsive scaffolds, and hierarchically structured materials that better recapitulate the complexity of native tissues. The integration of bioactive molecules, growth factors, and cell-instructive signals within nanofiber architectures has further enhanced their biomimetic properties, enabling more precise control over cellular behavior and tissue development.
The global research landscape shows a clear trajectory toward more sophisticated fabrication techniques beyond conventional electrospinning, including rotary jet spinning, solution blow spinning, and 3D bioprinting of nanofibers. These advances have expanded the potential applications of nanofiber scaffolds from simple tissue substitutes to complex organ-on-chip models and drug delivery systems.
The primary objectives of current research in biomimetic nanofiber scaffolds encompass several dimensions. First, improving the mechanical properties to match those of target tissues while maintaining appropriate porosity and degradation profiles. Second, enhancing biocompatibility and bioactivity through surface functionalization and incorporation of cell-signaling molecules. Third, developing scalable manufacturing processes that can translate laboratory successes into clinically viable products.
Another critical goal is to achieve precise spatial control over fiber orientation, alignment, and density to recreate the anisotropic nature of many native tissues. This directional organization is particularly important for tissues like muscle, tendon, and neural structures where cellular alignment directly influences functionality.
Looking forward, the field aims to develop "smart" nanofiber scaffolds capable of responding dynamically to cellular activities and environmental cues, potentially revolutionizing personalized medicine approaches. The ultimate technical objective remains the creation of fully functional, transplantable tissue constructs that can seamlessly integrate with the host and restore normal physiological function.
Market Analysis for Tissue Engineering Applications
The global tissue engineering market is experiencing robust growth, valued at approximately $12.8 billion in 2023 and projected to reach $31.2 billion by 2028, with a compound annual growth rate (CAGR) of 19.5%. This significant expansion is primarily driven by the increasing prevalence of chronic diseases, organ failure cases, and traumatic injuries requiring tissue replacement or regeneration solutions.
Biomimetic scaffolds with nanofiber architectures represent a particularly promising segment within this market, addressing critical needs in wound healing, orthopedics, cardiovascular applications, and neural tissue engineering. The demand for these advanced scaffolds is accelerating due to their superior ability to mimic the natural extracellular matrix (ECM), providing enhanced cell adhesion, proliferation, and differentiation compared to conventional scaffolds.
North America currently dominates the market with approximately 40% share, benefiting from substantial research funding, advanced healthcare infrastructure, and presence of key industry players. Europe follows with roughly 30% market share, while Asia-Pacific represents the fastest-growing region with annual growth exceeding 22%, driven by increasing healthcare expenditure, improving research capabilities, and favorable government initiatives in countries like China, Japan, and South Korea.
The orthopedic application segment constitutes the largest market share (35%) for nanofiber scaffolds, addressing the growing incidence of musculoskeletal disorders and sports injuries. Skin tissue engineering applications follow closely at 28%, responding to the rising prevalence of chronic wounds, burns, and diabetic ulcers. Cardiovascular applications represent 20% of the market, while neural tissue engineering, though smaller at 10%, demonstrates the highest growth potential.
End-user analysis reveals hospitals and surgical centers as primary consumers (45%), followed by research institutions (30%) and biotechnology companies (25%). This distribution highlights the dual nature of the market, serving both clinical applications and ongoing research endeavors.
Key market drivers include aging populations worldwide, increasing prevalence of lifestyle-related diseases, growing demand for organ transplantation alternatives, and rising investment in regenerative medicine research. However, market challenges persist, including high production costs, complex regulatory pathways, limited reimbursement policies, and technical challenges in scaling production while maintaining structural integrity and biocompatibility.
The competitive landscape features established players like Medtronic, Johnson & Johnson, and Smith & Nephew alongside innovative startups such as Nanofiber Solutions and Electrospinning Company, creating a dynamic market environment characterized by strategic partnerships, licensing agreements, and continuous technological advancement.
Biomimetic scaffolds with nanofiber architectures represent a particularly promising segment within this market, addressing critical needs in wound healing, orthopedics, cardiovascular applications, and neural tissue engineering. The demand for these advanced scaffolds is accelerating due to their superior ability to mimic the natural extracellular matrix (ECM), providing enhanced cell adhesion, proliferation, and differentiation compared to conventional scaffolds.
North America currently dominates the market with approximately 40% share, benefiting from substantial research funding, advanced healthcare infrastructure, and presence of key industry players. Europe follows with roughly 30% market share, while Asia-Pacific represents the fastest-growing region with annual growth exceeding 22%, driven by increasing healthcare expenditure, improving research capabilities, and favorable government initiatives in countries like China, Japan, and South Korea.
The orthopedic application segment constitutes the largest market share (35%) for nanofiber scaffolds, addressing the growing incidence of musculoskeletal disorders and sports injuries. Skin tissue engineering applications follow closely at 28%, responding to the rising prevalence of chronic wounds, burns, and diabetic ulcers. Cardiovascular applications represent 20% of the market, while neural tissue engineering, though smaller at 10%, demonstrates the highest growth potential.
End-user analysis reveals hospitals and surgical centers as primary consumers (45%), followed by research institutions (30%) and biotechnology companies (25%). This distribution highlights the dual nature of the market, serving both clinical applications and ongoing research endeavors.
Key market drivers include aging populations worldwide, increasing prevalence of lifestyle-related diseases, growing demand for organ transplantation alternatives, and rising investment in regenerative medicine research. However, market challenges persist, including high production costs, complex regulatory pathways, limited reimbursement policies, and technical challenges in scaling production while maintaining structural integrity and biocompatibility.
The competitive landscape features established players like Medtronic, Johnson & Johnson, and Smith & Nephew alongside innovative startups such as Nanofiber Solutions and Electrospinning Company, creating a dynamic market environment characterized by strategic partnerships, licensing agreements, and continuous technological advancement.
Current Challenges in Nanofiber Scaffold Technology
Despite significant advancements in nanofiber scaffold technology for biomimetic applications, several critical challenges continue to impede widespread clinical translation and commercial viability. One of the primary obstacles remains the scalable manufacturing of nanofiber scaffolds with consistent quality and reproducible architectures. Current electrospinning techniques, while versatile, often suffer from batch-to-batch variations that compromise structural integrity and biological performance.
The precise control of fiber orientation, alignment, and three-dimensional architecture represents another substantial hurdle. While random fiber networks are relatively straightforward to produce, creating biomimetic scaffolds that accurately replicate the complex hierarchical structures of native tissues demands sophisticated fabrication approaches that are not yet fully realized at industrial scale.
Material selection presents a multifaceted challenge, as the ideal scaffold must balance mechanical properties, degradation kinetics, and biocompatibility. Synthetic polymers offer tunable mechanical characteristics but often lack the biological recognition sites necessary for optimal cell-material interactions. Conversely, natural polymers provide excellent biocompatibility but frequently exhibit poor mechanical stability and rapid degradation rates.
The incorporation of bioactive molecules and growth factors into nanofiber scaffolds without compromising their biological activity during fabrication processes remains problematic. Harsh solvents, high voltages, and mechanical stresses during electrospinning can denature proteins and reduce the efficacy of incorporated biomolecules.
Porosity control and cellular infiltration represent significant technical barriers. While nanofiber architectures inherently possess high surface area-to-volume ratios, the dense packing of fibers often restricts cell migration into the scaffold interior, limiting the formation of fully integrated three-dimensional tissues.
Sterilization methods compatible with nanofiber scaffolds constitute another challenge, as conventional techniques like autoclaving or gamma irradiation can alter material properties, degradation profiles, and the activity of incorporated bioactive components.
Regulatory hurdles further complicate the path to clinical implementation. The complex and often proprietary nature of nanofiber scaffold fabrication processes creates challenges for standardization, quality control, and regulatory approval, particularly for scaffolds incorporating multiple materials or bioactive components.
Cost-effectiveness remains a significant concern, as current production methods for high-quality nanofiber scaffolds are labor-intensive and material-inefficient, resulting in prohibitively expensive products for widespread clinical adoption.
The precise control of fiber orientation, alignment, and three-dimensional architecture represents another substantial hurdle. While random fiber networks are relatively straightforward to produce, creating biomimetic scaffolds that accurately replicate the complex hierarchical structures of native tissues demands sophisticated fabrication approaches that are not yet fully realized at industrial scale.
Material selection presents a multifaceted challenge, as the ideal scaffold must balance mechanical properties, degradation kinetics, and biocompatibility. Synthetic polymers offer tunable mechanical characteristics but often lack the biological recognition sites necessary for optimal cell-material interactions. Conversely, natural polymers provide excellent biocompatibility but frequently exhibit poor mechanical stability and rapid degradation rates.
The incorporation of bioactive molecules and growth factors into nanofiber scaffolds without compromising their biological activity during fabrication processes remains problematic. Harsh solvents, high voltages, and mechanical stresses during electrospinning can denature proteins and reduce the efficacy of incorporated biomolecules.
Porosity control and cellular infiltration represent significant technical barriers. While nanofiber architectures inherently possess high surface area-to-volume ratios, the dense packing of fibers often restricts cell migration into the scaffold interior, limiting the formation of fully integrated three-dimensional tissues.
Sterilization methods compatible with nanofiber scaffolds constitute another challenge, as conventional techniques like autoclaving or gamma irradiation can alter material properties, degradation profiles, and the activity of incorporated bioactive components.
Regulatory hurdles further complicate the path to clinical implementation. The complex and often proprietary nature of nanofiber scaffold fabrication processes creates challenges for standardization, quality control, and regulatory approval, particularly for scaffolds incorporating multiple materials or bioactive components.
Cost-effectiveness remains a significant concern, as current production methods for high-quality nanofiber scaffolds are labor-intensive and material-inefficient, resulting in prohibitively expensive products for widespread clinical adoption.
Current Fabrication Approaches for Nanofiber Architectures
01 Electrospun nanofiber scaffolds for tissue engineering
Electrospinning technology is used to create biomimetic nanofiber scaffolds that mimic the natural extracellular matrix. These scaffolds provide structural support for cell adhesion, proliferation, and differentiation in tissue engineering applications. The nanoscale architecture promotes cell-material interactions and enhances tissue regeneration by mimicking the native tissue environment.- Electrospun nanofiber scaffolds for tissue engineering: Electrospinning technology is used to create biomimetic nanofiber scaffolds that mimic the natural extracellular matrix. These scaffolds provide structural support for cell adhesion, proliferation, and differentiation in tissue engineering applications. The nanoscale architecture promotes cell-material interactions and enhances tissue regeneration by mimicking the native tissue environment. Various polymers can be electrospun to create scaffolds with controlled fiber diameter, porosity, and mechanical properties.
- Self-assembling peptide nanofiber scaffolds: Self-assembling peptides can form nanofiber networks that serve as biomimetic scaffolds for tissue regeneration. These peptides spontaneously assemble into nanofibers under physiological conditions, creating hydrogels that mimic the natural extracellular matrix. The resulting scaffolds provide a 3D microenvironment for cell growth and can be functionalized with bioactive motifs to enhance cell attachment and differentiation. These scaffolds are particularly useful for neural tissue engineering and wound healing applications.
- Composite nanofiber scaffolds with bioactive components: Composite nanofiber scaffolds incorporate bioactive components such as growth factors, drugs, or nanoparticles to enhance their biological functionality. These scaffolds combine the structural advantages of nanofiber architectures with the biological activity of incorporated components. The bioactive molecules can be released in a controlled manner to guide cell behavior and tissue formation. Various fabrication methods allow for the incorporation of bioactive components either within the nanofibers or as surface modifications.
- Aligned nanofiber scaffolds for directional tissue growth: Aligned nanofiber scaffolds provide topographical cues that guide directional cell growth and tissue formation. These scaffolds are particularly important for tissues with aligned structures, such as neural tissue, muscle, and tendons. The alignment of nanofibers can be achieved through various fabrication techniques, including rotating collectors during electrospinning or magnetic field alignment. The aligned architecture promotes cell elongation along the fiber direction and enhances the mechanical properties of the resulting tissue.
- Stimuli-responsive nanofiber scaffolds: Stimuli-responsive nanofiber scaffolds can change their properties in response to external stimuli such as temperature, pH, light, or electrical signals. These smart scaffolds provide dynamic microenvironments that can better mimic the changing conditions of natural tissues. The responsive behavior can be used to control cell behavior, drug release, or scaffold degradation. Applications include controlled drug delivery systems, tissue engineering constructs that adapt to the healing process, and biomimetic actuators for soft robotics.
02 Composite nanofiber scaffolds with bioactive components
Biomimetic scaffolds can be enhanced by incorporating bioactive components such as growth factors, peptides, or minerals into the nanofiber architecture. These composite scaffolds provide both structural support and biochemical cues to guide cell behavior and tissue formation. The controlled release of bioactive molecules from the nanofiber matrix creates a dynamic microenvironment that promotes tissue regeneration and healing.Expand Specific Solutions03 Aligned nanofiber architectures for directional tissue growth
Specially designed nanofiber scaffolds with aligned fiber orientation can guide directional cell growth and tissue formation. These aligned architectures are particularly useful for engineering tissues with anisotropic properties, such as muscle, nerve, and tendon. The aligned nanofibers provide contact guidance for cells, influencing their morphology, migration, and differentiation in a direction-dependent manner.Expand Specific Solutions04 Multi-layered nanofiber scaffolds with hierarchical structures
Biomimetic scaffolds with multi-layered or hierarchical nanofiber architectures better replicate the complex structural organization of native tissues. These scaffolds feature varying fiber diameters, orientations, and compositions across different layers, mimicking the hierarchical structure of natural tissues. The multi-scale architecture provides mechanical stability while supporting cellular functions at different organizational levels.Expand Specific Solutions05 Stimuli-responsive nanofiber scaffolds
Advanced biomimetic scaffolds incorporate stimuli-responsive nanofiber architectures that can change their properties in response to external stimuli such as temperature, pH, or mechanical forces. These smart scaffolds can dynamically adapt to the changing needs of the regenerating tissue, providing temporal control over scaffold properties. The responsive behavior enables on-demand release of bioactive agents or changes in scaffold mechanics to guide tissue development.Expand Specific Solutions
Leading Research Institutions and Companies in Biomimetic Materials
Biomimetic scaffolds with nanofiber architectures represent an emerging field in tissue engineering, currently in the growth phase with increasing market adoption. The global market for these scaffolds is expanding rapidly, driven by applications in regenerative medicine and drug delivery systems. Technologically, the field is advancing from early development to commercial maturity, with academic institutions like Zhejiang University, Tsinghua University, and Columbia University leading fundamental research, while companies such as Ultra Small Fibers LLC and Zhongkesino Biomaterial Technology are commercializing applications. Research collaborations between universities and industry partners are accelerating innovation, particularly in areas of controlled fiber architecture and biocompatibility, positioning this technology for significant healthcare impact in the coming decade.
Zhejiang University
Technical Solution: Zhejiang University has developed advanced biomimetic nanofiber scaffolds using a combination of electrospinning, phase separation, and self-assembly techniques. Their proprietary "hierarchical electrospinning" technology creates multi-scale fibrous structures that mimic the complex architecture of native tissues. They've pioneered the incorporation of tissue-specific extracellular matrix components derived from decellularized tissues into synthetic nanofibers, creating hybrid scaffolds with enhanced bioactivity. Their research has yielded nanofiber scaffolds with spatially controlled mechanical properties that can guide cell migration and differentiation through mechanotransduction pathways. Zhejiang researchers have developed innovative surface modification techniques to create nanofibers with biomolecule gradients that mimic developmental cues. Their recent work includes magnetically responsive nanofiber scaffolds that can be remotely manipulated to apply mechanical stimulation to cells, enhancing tissue maturation. They've also pioneered the integration of microfluidic channels within nanofiber scaffolds to improve nutrient transport in thick engineered tissues.
Strengths: Excellent integration of natural ECM components with synthetic materials; sophisticated control over spatial organization of biochemical and mechanical properties; innovative approaches to creating dynamic, stimuli-responsive scaffolds. Weaknesses: Some technologies remain at early stages of development; complex manufacturing processes may present challenges for standardization and scale-up; limited long-term in vivo validation for some newer scaffold designs.
University of Washington
Technical Solution: University of Washington has developed innovative biomimetic nanofiber scaffolds using a combination of electrospinning and microfluidic fabrication techniques. Their patented "controlled fiber architecture" technology enables precise control over fiber diameter, orientation, and spatial arrangement to mimic tissue-specific extracellular matrix structures. They've pioneered the development of self-healing nanofiber scaffolds incorporating dynamic chemical bonds that can repair microdamage, extending scaffold lifetime and functionality. Their research has yielded gradient nanofiber scaffolds with spatially varying mechanical and biochemical properties for interfacial tissue engineering applications, particularly for musculoskeletal tissues. UW researchers have developed innovative approaches to creating vascularized nanofiber scaffolds by incorporating sacrificial fibers that create channels for blood vessel formation. Their recent work includes electrically conductive nanofiber scaffolds incorporating graphene and conductive polymers for neural tissue engineering and biosensing applications. They've also developed biodegradable nanofiber scaffolds with programmable degradation rates that match the pace of native tissue formation.
Strengths: Excellent control over scaffold architecture and mechanical properties; innovative approaches to creating dynamic, responsive scaffolds; strong focus on translational applications with several technologies advancing toward clinical testing. Weaknesses: Some technologies require specialized equipment limiting widespread adoption; complex manufacturing processes may present challenges for standardization; higher costs associated with more sophisticated scaffold designs.
Key Patents and Breakthroughs in Biomimetic Scaffold Design
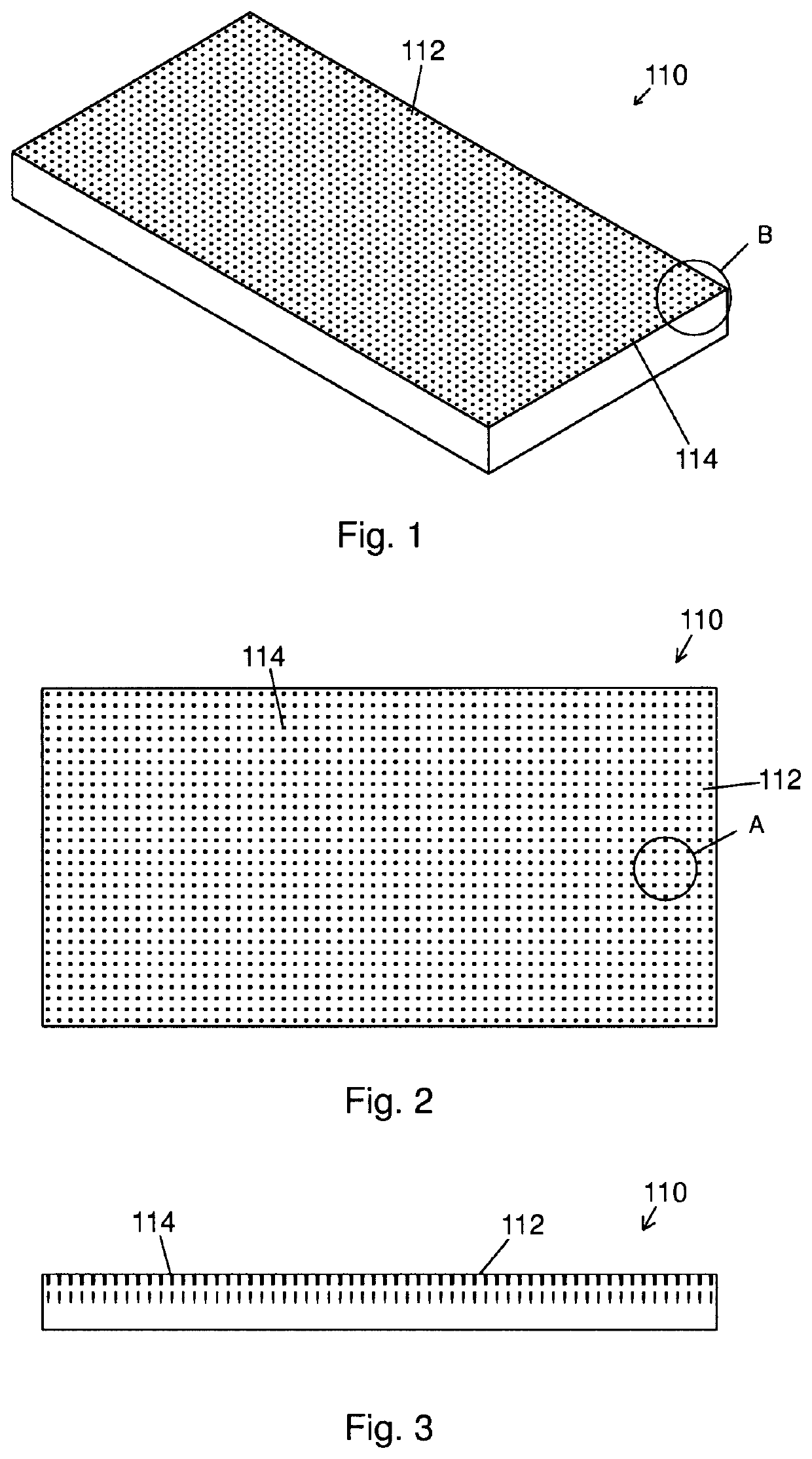
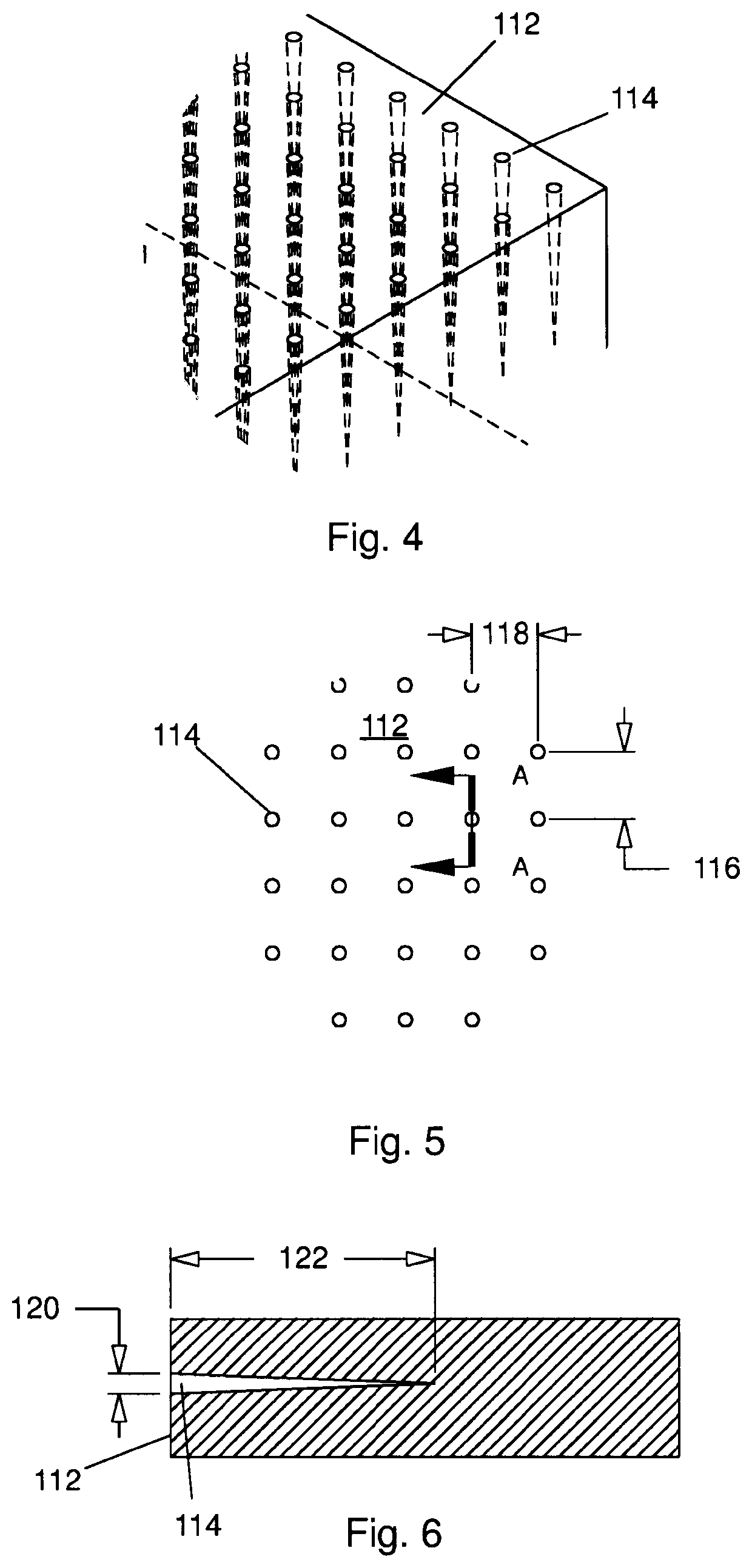
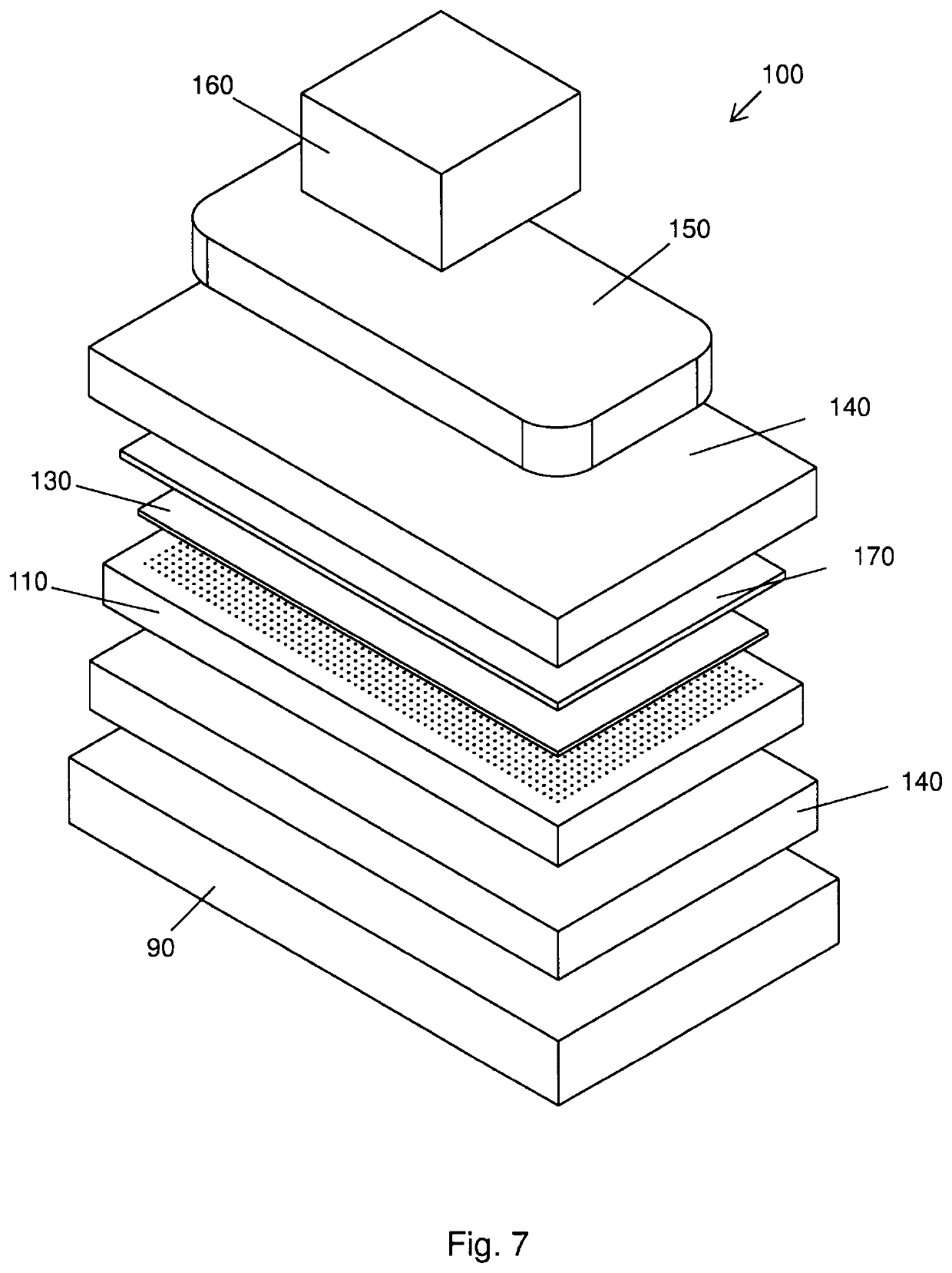
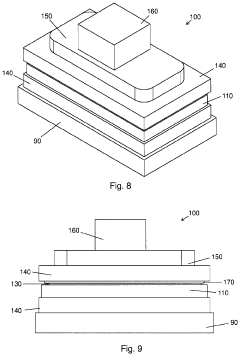
Biomimetic nanofiber tissue scaffolds
PatentActiveUS20220347342A1
Innovation
- The development of biomimetic tissue scaffolds with patterned matrices of nanofibers that mimic the tendril arrays of the extracellular matrix, featuring nanofibers of finite length and irregular shapes, anchored to a stiff matrix, allowing for secure cell attachment and the creation of shear stresses, thereby influencing cell behavior and differentiation.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development of biomimetic scaffolds with nanofiber architectures for tissue engineering and regenerative medicine applications. The interaction between these scaffolds and biological systems must be thoroughly evaluated to ensure patient safety and therapeutic efficacy.
The biocompatibility assessment of nanofiber scaffolds begins with material selection. Synthetic polymers such as poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyurethane (PU) have demonstrated acceptable biocompatibility profiles, while natural polymers including collagen, silk fibroin, and chitosan often exhibit superior cell recognition properties but may present immunogenicity concerns. The processing methods for nanofiber production, particularly electrospinning, may introduce residual solvents or cross-linking agents that require rigorous purification protocols.
Cytotoxicity testing represents the first tier of biocompatibility evaluation, typically involving direct contact assays with relevant cell lines to assess cell viability, proliferation, and morphological changes. More comprehensive in vitro assessments examine cellular adhesion, migration, and differentiation on nanofiber scaffolds. The architectural features of nanofibers, including fiber diameter, orientation, and porosity, significantly influence cellular responses and must be optimized for specific tissue applications.
Inflammatory responses to nanofiber scaffolds constitute a critical safety consideration. The surface chemistry and topography of nanofibers can modulate macrophage polarization toward pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes, thereby influencing the foreign body response and subsequent tissue integration. Nanoscale features may also trigger specific immune recognition pathways that differ from conventional biomaterials.
Long-term safety evaluations must address the degradation kinetics and metabolic fate of nanofiber scaffolds. The breakdown products should be non-toxic and efficiently cleared from the body without accumulation in vital organs. Particular attention must be paid to potential nanomaterial-specific toxicity mechanisms, including oxidative stress induction, genotoxicity, and disruption of cellular membranes or organelles.
Regulatory considerations for nanofiber scaffolds are evolving as regulatory agencies develop frameworks for nanomedicine evaluation. Current approaches typically require additional characterization and safety testing beyond conventional biomaterials, including nanoparticle release assessments during degradation and potential translocation studies. International standards organizations are working to establish standardized protocols for nanomaterial safety evaluation in biomedical applications.
Advanced imaging techniques and analytical methods are increasingly employed to monitor the in vivo performance and safety of nanofiber scaffolds, including non-invasive tracking of scaffold degradation and tissue integration through time. These approaches provide valuable insights into the dynamic interactions between nanofiber architectures and biological systems, supporting the development of safer and more effective biomimetic scaffolds.
The biocompatibility assessment of nanofiber scaffolds begins with material selection. Synthetic polymers such as poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyurethane (PU) have demonstrated acceptable biocompatibility profiles, while natural polymers including collagen, silk fibroin, and chitosan often exhibit superior cell recognition properties but may present immunogenicity concerns. The processing methods for nanofiber production, particularly electrospinning, may introduce residual solvents or cross-linking agents that require rigorous purification protocols.
Cytotoxicity testing represents the first tier of biocompatibility evaluation, typically involving direct contact assays with relevant cell lines to assess cell viability, proliferation, and morphological changes. More comprehensive in vitro assessments examine cellular adhesion, migration, and differentiation on nanofiber scaffolds. The architectural features of nanofibers, including fiber diameter, orientation, and porosity, significantly influence cellular responses and must be optimized for specific tissue applications.
Inflammatory responses to nanofiber scaffolds constitute a critical safety consideration. The surface chemistry and topography of nanofibers can modulate macrophage polarization toward pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes, thereby influencing the foreign body response and subsequent tissue integration. Nanoscale features may also trigger specific immune recognition pathways that differ from conventional biomaterials.
Long-term safety evaluations must address the degradation kinetics and metabolic fate of nanofiber scaffolds. The breakdown products should be non-toxic and efficiently cleared from the body without accumulation in vital organs. Particular attention must be paid to potential nanomaterial-specific toxicity mechanisms, including oxidative stress induction, genotoxicity, and disruption of cellular membranes or organelles.
Regulatory considerations for nanofiber scaffolds are evolving as regulatory agencies develop frameworks for nanomedicine evaluation. Current approaches typically require additional characterization and safety testing beyond conventional biomaterials, including nanoparticle release assessments during degradation and potential translocation studies. International standards organizations are working to establish standardized protocols for nanomaterial safety evaluation in biomedical applications.
Advanced imaging techniques and analytical methods are increasingly employed to monitor the in vivo performance and safety of nanofiber scaffolds, including non-invasive tracking of scaffold degradation and tissue integration through time. These approaches provide valuable insights into the dynamic interactions between nanofiber architectures and biological systems, supporting the development of safer and more effective biomimetic scaffolds.
Regulatory Pathway for Clinical Translation
The regulatory pathway for biomimetic scaffolds with nanofiber architectures involves navigating complex approval processes across different global regulatory bodies. In the United States, the FDA typically classifies these scaffolds as combination products under the Center for Biologics Evaluation and Research (CBER) or the Center for Devices and Radiological Health (CDRH), depending on their primary mode of action. The regulatory journey begins with preclinical testing, focusing on biocompatibility, degradation profiles, and mechanical properties specific to nanofiber architectures.
For clinical translation, developers must submit an Investigational New Drug (IND) application or Investigational Device Exemption (IDE), supported by comprehensive preclinical data demonstrating safety and preliminary efficacy. The unique nanoscale features of biomimetic scaffolds often require specialized characterization methods to satisfy regulatory requirements, including detailed analysis of fiber diameter consistency, porosity, and surface topography.
European regulatory pathways differ significantly, with the European Medicines Agency (EMA) classifying most biomimetic scaffolds under the Medical Device Regulation (MDR) or Advanced Therapy Medicinal Products (ATMP) framework if they contain cellular components. The CE marking process requires conformity assessment by Notified Bodies, with particular attention to risk management documentation addressing potential nanofiber-specific concerns such as degradation byproducts and structural integrity over time.
Quality control represents a critical regulatory challenge for nanofiber scaffolds. Manufacturers must establish robust quality management systems with validated processes for consistent production of nanofiber architectures. This includes implementing in-process controls to monitor fiber formation, orientation, and dimensional stability, which directly impact the scaffold's biomimetic properties and clinical performance.
Regulatory agencies increasingly require post-market surveillance plans tailored to the unique characteristics of biomimetic scaffolds. These plans must address long-term integration with host tissue, potential for delayed immune responses, and maintenance of mechanical properties throughout the intended lifespan of the implant. The FDA's Total Product Life Cycle approach and the EU's EUDAMED database requirements have created more stringent post-approval monitoring expectations.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually establishing consistent standards for nanofiber-based medical products. However, significant regional variations persist, necessitating customized regulatory strategies for global market access. Developers should engage early with regulatory authorities through pre-submission meetings to address novel aspects of their nanofiber technologies and establish appropriate testing protocols.
For clinical translation, developers must submit an Investigational New Drug (IND) application or Investigational Device Exemption (IDE), supported by comprehensive preclinical data demonstrating safety and preliminary efficacy. The unique nanoscale features of biomimetic scaffolds often require specialized characterization methods to satisfy regulatory requirements, including detailed analysis of fiber diameter consistency, porosity, and surface topography.
European regulatory pathways differ significantly, with the European Medicines Agency (EMA) classifying most biomimetic scaffolds under the Medical Device Regulation (MDR) or Advanced Therapy Medicinal Products (ATMP) framework if they contain cellular components. The CE marking process requires conformity assessment by Notified Bodies, with particular attention to risk management documentation addressing potential nanofiber-specific concerns such as degradation byproducts and structural integrity over time.
Quality control represents a critical regulatory challenge for nanofiber scaffolds. Manufacturers must establish robust quality management systems with validated processes for consistent production of nanofiber architectures. This includes implementing in-process controls to monitor fiber formation, orientation, and dimensional stability, which directly impact the scaffold's biomimetic properties and clinical performance.
Regulatory agencies increasingly require post-market surveillance plans tailored to the unique characteristics of biomimetic scaffolds. These plans must address long-term integration with host tissue, potential for delayed immune responses, and maintenance of mechanical properties throughout the intended lifespan of the implant. The FDA's Total Product Life Cycle approach and the EU's EUDAMED database requirements have created more stringent post-approval monitoring expectations.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually establishing consistent standards for nanofiber-based medical products. However, significant regional variations persist, necessitating customized regulatory strategies for global market access. Developers should engage early with regulatory authorities through pre-submission meetings to address novel aspects of their nanofiber technologies and establish appropriate testing protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!