How biomimetic scaffolds influence vascularization in tissue engineering
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomimetic Scaffolds and Vascularization Background
Tissue engineering represents a revolutionary approach to regenerative medicine, aiming to restore, maintain, or enhance tissue function through the development of biological substitutes. At the core of this discipline lies the critical challenge of vascularization—the formation of blood vessel networks essential for nutrient delivery and waste removal in engineered tissues. Traditional scaffolds have faced significant limitations in supporting adequate vascularization, resulting in poor cell viability and tissue integration, particularly in constructs exceeding 200 micrometers in thickness.
Biomimetic scaffolds have emerged as a promising solution to this fundamental challenge. These advanced structures are designed to emulate the natural extracellular matrix (ECM) in terms of composition, architecture, and biological functionality. The concept of biomimicry in tissue engineering dates back to the early 2000s but has gained substantial momentum over the past decade with advancements in fabrication technologies and deeper understanding of cell-matrix interactions.
The evolution of biomimetic scaffolds has progressed from simple porous structures to sophisticated designs incorporating specific topographical features, mechanical properties, and bioactive components that actively promote vascularization. Early approaches focused primarily on material selection and porosity, while contemporary designs integrate multiple biophysical and biochemical cues to orchestrate complex cellular behaviors including angiogenesis—the formation of new blood vessels from existing vasculature.
Vascularization in tissue engineering encompasses several interconnected processes: vasculogenesis (de novo formation of blood vessels), angiogenesis (sprouting of new vessels from existing ones), and inosculation (connection of engineered vasculature with host circulation). Biomimetic scaffolds can influence each of these processes through various mechanisms, including the presentation of pro-angiogenic factors, creation of hypoxic microenvironments, and provision of physical guidance cues for endothelial cell migration and organization.
The field has witnessed significant technological breakthroughs, including the development of 3D bioprinting techniques capable of creating pre-vascularized constructs, decellularization methods that preserve native vascular networks, and smart materials that respond dynamically to cellular activities. These innovations have collectively expanded the possibilities for engineering vascularized tissues of increasing complexity and clinical relevance.
Despite these advances, substantial challenges remain in achieving fully functional vascularization that recapitulates the hierarchical organization and physiological performance of native vasculature. The integration of engineered vascular networks with host circulation represents a particularly critical hurdle for clinical translation. Current research increasingly focuses on multi-disciplinary approaches combining advanced biomaterials, controlled release systems, and cellular co-culture strategies to address these challenges.
Biomimetic scaffolds have emerged as a promising solution to this fundamental challenge. These advanced structures are designed to emulate the natural extracellular matrix (ECM) in terms of composition, architecture, and biological functionality. The concept of biomimicry in tissue engineering dates back to the early 2000s but has gained substantial momentum over the past decade with advancements in fabrication technologies and deeper understanding of cell-matrix interactions.
The evolution of biomimetic scaffolds has progressed from simple porous structures to sophisticated designs incorporating specific topographical features, mechanical properties, and bioactive components that actively promote vascularization. Early approaches focused primarily on material selection and porosity, while contemporary designs integrate multiple biophysical and biochemical cues to orchestrate complex cellular behaviors including angiogenesis—the formation of new blood vessels from existing vasculature.
Vascularization in tissue engineering encompasses several interconnected processes: vasculogenesis (de novo formation of blood vessels), angiogenesis (sprouting of new vessels from existing ones), and inosculation (connection of engineered vasculature with host circulation). Biomimetic scaffolds can influence each of these processes through various mechanisms, including the presentation of pro-angiogenic factors, creation of hypoxic microenvironments, and provision of physical guidance cues for endothelial cell migration and organization.
The field has witnessed significant technological breakthroughs, including the development of 3D bioprinting techniques capable of creating pre-vascularized constructs, decellularization methods that preserve native vascular networks, and smart materials that respond dynamically to cellular activities. These innovations have collectively expanded the possibilities for engineering vascularized tissues of increasing complexity and clinical relevance.
Despite these advances, substantial challenges remain in achieving fully functional vascularization that recapitulates the hierarchical organization and physiological performance of native vasculature. The integration of engineered vascular networks with host circulation represents a particularly critical hurdle for clinical translation. Current research increasingly focuses on multi-disciplinary approaches combining advanced biomaterials, controlled release systems, and cellular co-culture strategies to address these challenges.
Market Analysis of Vascularized Tissue Engineering
The global market for vascularized tissue engineering is experiencing significant growth, driven by increasing prevalence of chronic diseases, rising demand for organ transplantation, and advancements in biomimetic scaffold technologies. Currently valued at approximately $12.3 billion, this market is projected to reach $45.6 billion by 2030, representing a compound annual growth rate of 15.7% during the forecast period.
Demand for vascularized tissue engineering solutions is primarily concentrated in North America and Europe, which collectively account for over 60% of the global market share. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and Japan leading regional development due to increasing healthcare expenditure and government initiatives supporting regenerative medicine research.
The application landscape for vascularized tissue engineering is diverse, with cardiovascular applications representing the largest segment (38% market share), followed by orthopedic (22%), skin and wound healing (18%), and neurological applications (12%). The remaining 10% encompasses various applications including hepatic, renal, and pancreatic tissue engineering.
Key market drivers include the persistent organ donation shortage, with waiting lists for transplants growing at approximately 7% annually in developed countries. Additionally, the aging global population and associated increase in degenerative diseases create substantial demand for tissue-engineered alternatives. The cost-effectiveness of tissue-engineered products compared to long-term patient care for chronic conditions further stimulates market growth.
Regulatory environments significantly impact market dynamics, with the FDA and EMA establishing clearer pathways for approval of tissue-engineered products. This regulatory clarity has encouraged increased investment in the sector, with venture capital funding for vascularized tissue engineering startups reaching $3.2 billion in 2022, a 24% increase from the previous year.
Reimbursement policies remain a critical challenge, as many healthcare systems have not yet established comprehensive coverage for tissue-engineered products. However, positive clinical outcomes demonstrating long-term cost savings are gradually influencing policy changes in favor of these technologies.
The market is witnessing a shift toward personalized medicine approaches, with patient-specific biomimetic scaffolds gaining traction. This trend is supported by advancements in 3D bioprinting technologies and increasing integration of artificial intelligence in scaffold design optimization, creating new market opportunities for companies with expertise in these convergent technologies.
Demand for vascularized tissue engineering solutions is primarily concentrated in North America and Europe, which collectively account for over 60% of the global market share. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and Japan leading regional development due to increasing healthcare expenditure and government initiatives supporting regenerative medicine research.
The application landscape for vascularized tissue engineering is diverse, with cardiovascular applications representing the largest segment (38% market share), followed by orthopedic (22%), skin and wound healing (18%), and neurological applications (12%). The remaining 10% encompasses various applications including hepatic, renal, and pancreatic tissue engineering.
Key market drivers include the persistent organ donation shortage, with waiting lists for transplants growing at approximately 7% annually in developed countries. Additionally, the aging global population and associated increase in degenerative diseases create substantial demand for tissue-engineered alternatives. The cost-effectiveness of tissue-engineered products compared to long-term patient care for chronic conditions further stimulates market growth.
Regulatory environments significantly impact market dynamics, with the FDA and EMA establishing clearer pathways for approval of tissue-engineered products. This regulatory clarity has encouraged increased investment in the sector, with venture capital funding for vascularized tissue engineering startups reaching $3.2 billion in 2022, a 24% increase from the previous year.
Reimbursement policies remain a critical challenge, as many healthcare systems have not yet established comprehensive coverage for tissue-engineered products. However, positive clinical outcomes demonstrating long-term cost savings are gradually influencing policy changes in favor of these technologies.
The market is witnessing a shift toward personalized medicine approaches, with patient-specific biomimetic scaffolds gaining traction. This trend is supported by advancements in 3D bioprinting technologies and increasing integration of artificial intelligence in scaffold design optimization, creating new market opportunities for companies with expertise in these convergent technologies.
Current Challenges in Scaffold-Induced Vascularization
Despite significant advancements in biomimetic scaffold design for tissue engineering, vascularization remains one of the most critical challenges limiting clinical translation. Current scaffolds struggle to support the formation of functional vascular networks capable of sustaining large-scale engineered tissues. The primary obstacle lies in replicating the complex hierarchical structure of native vasculature, which ranges from large vessels to intricate capillary beds with diameters as small as 5-10 μm.
Oxygen diffusion limitations present a fundamental constraint, as cells located more than 200 μm from a blood vessel face hypoxic conditions and eventual necrosis. This diffusion barrier severely restricts the size of viable engineered tissues without adequate vascularization strategies. While various approaches have been developed to enhance vascularization, each presents specific challenges that impede widespread implementation.
Pre-vascularization techniques, which involve creating vessel networks prior to implantation, often fail to achieve proper integration with host vasculature. The anastomosis between engineered and native vessels occurs too slowly to prevent core necrosis in larger constructs. Additionally, maintaining the stability and functionality of pre-formed vessels during implantation remains problematic.
Growth factor delivery systems face challenges related to spatiotemporal control. Current methods typically result in burst release profiles rather than the sustained, gradient-based delivery needed to mimic natural angiogenic processes. Furthermore, the high cost and instability of growth factors like VEGF and bFGF limit their practical application in clinical settings.
Material selection presents another significant hurdle. While natural materials offer excellent biocompatibility and cell-recognition sites, they often lack mechanical stability and exhibit batch-to-batch variability. Synthetic materials provide better mechanical properties and reproducibility but frequently lack the biological cues necessary to guide proper vascular development.
Microarchitectural design of scaffolds faces technical limitations in manufacturing precision. Creating biomimetic structures with features spanning multiple scale levels—from macro to nano—remains challenging with current fabrication technologies. The integration of different manufacturing techniques often results in compromised structural integrity or bioactivity.
Regulatory and scalability issues further complicate clinical translation. Complex vascularized scaffolds face stringent regulatory scrutiny regarding safety, reproducibility, and quality control. Additionally, the labor-intensive and time-consuming nature of current fabrication methods makes large-scale production economically unfeasible for widespread clinical application.
Oxygen diffusion limitations present a fundamental constraint, as cells located more than 200 μm from a blood vessel face hypoxic conditions and eventual necrosis. This diffusion barrier severely restricts the size of viable engineered tissues without adequate vascularization strategies. While various approaches have been developed to enhance vascularization, each presents specific challenges that impede widespread implementation.
Pre-vascularization techniques, which involve creating vessel networks prior to implantation, often fail to achieve proper integration with host vasculature. The anastomosis between engineered and native vessels occurs too slowly to prevent core necrosis in larger constructs. Additionally, maintaining the stability and functionality of pre-formed vessels during implantation remains problematic.
Growth factor delivery systems face challenges related to spatiotemporal control. Current methods typically result in burst release profiles rather than the sustained, gradient-based delivery needed to mimic natural angiogenic processes. Furthermore, the high cost and instability of growth factors like VEGF and bFGF limit their practical application in clinical settings.
Material selection presents another significant hurdle. While natural materials offer excellent biocompatibility and cell-recognition sites, they often lack mechanical stability and exhibit batch-to-batch variability. Synthetic materials provide better mechanical properties and reproducibility but frequently lack the biological cues necessary to guide proper vascular development.
Microarchitectural design of scaffolds faces technical limitations in manufacturing precision. Creating biomimetic structures with features spanning multiple scale levels—from macro to nano—remains challenging with current fabrication technologies. The integration of different manufacturing techniques often results in compromised structural integrity or bioactivity.
Regulatory and scalability issues further complicate clinical translation. Complex vascularized scaffolds face stringent regulatory scrutiny regarding safety, reproducibility, and quality control. Additionally, the labor-intensive and time-consuming nature of current fabrication methods makes large-scale production economically unfeasible for widespread clinical application.
State-of-the-Art Biomimetic Vascularization Strategies
01 Natural polymer-based biomimetic scaffolds for vascularization
Natural polymers such as collagen, fibrin, and hyaluronic acid are used to create biomimetic scaffolds that promote vascularization. These materials closely mimic the extracellular matrix of native tissues, providing appropriate biochemical and mechanical cues for endothelial cell adhesion, migration, and blood vessel formation. The natural composition facilitates better integration with host tissue and reduces immune response, making them ideal for tissue engineering applications requiring robust vascular networks.- Natural polymer-based biomimetic scaffolds for vascularization: Natural polymers such as collagen, fibrin, and hyaluronic acid are used to create biomimetic scaffolds that promote vascularization. These materials closely mimic the extracellular matrix of native tissues, providing appropriate biochemical cues and mechanical properties that support cell adhesion, migration, and blood vessel formation. The natural composition facilitates better integration with host tissue and reduces immune response, making them ideal for tissue engineering applications requiring robust vascularization.
- Microfluidic systems for vascularized tissue constructs: Microfluidic technologies are employed to create perfusable vascular networks within biomimetic scaffolds. These systems enable precise control over the spatial arrangement of channels that mimic blood vessels, allowing for nutrient delivery and waste removal throughout engineered tissues. By incorporating endothelial cells into these microfluidic channels, functional vascular networks can be established that support the viability of larger tissue constructs. This approach addresses the critical challenge of oxygen diffusion limitations in thick engineered tissues.
- Growth factor delivery systems for enhanced vascularization: Controlled release systems for angiogenic growth factors such as VEGF, bFGF, and PDGF are incorporated into biomimetic scaffolds to stimulate blood vessel formation. These delivery systems can be designed to provide sustained release of growth factors over time or to respond to specific cellular cues. By precisely controlling the spatial and temporal presentation of these bioactive molecules, vascularization can be directed and enhanced within the scaffold, leading to improved tissue integration and functionality.
- 3D bioprinting of vascularized tissue scaffolds: Advanced 3D bioprinting techniques are utilized to fabricate complex biomimetic scaffolds with pre-designed vascular architectures. These methods allow for precise positioning of multiple cell types, biomaterials, and bioactive factors in a spatially controlled manner. By incorporating sacrificial materials or direct printing of vessel-like structures, interconnected vascular networks can be created within the scaffold. This technology enables the production of tissue constructs with hierarchical vascular structures that more closely resemble native tissues.
- Cell co-culture strategies for scaffold vascularization: Co-culture systems involving endothelial cells with supporting cell types such as fibroblasts, mesenchymal stem cells, or pericytes are employed to promote vascularization in biomimetic scaffolds. These supporting cells provide paracrine factors and physical interactions that enhance endothelial cell survival, migration, and tube formation. The synergistic interactions between different cell types create a more physiologically relevant microenvironment that accelerates the formation of stable and functional blood vessels within the engineered tissue constructs.
02 Microfluidic approaches for vascularized scaffold fabrication
Microfluidic technologies enable the creation of precisely controlled vascular networks within biomimetic scaffolds. These approaches allow for the formation of perfusable channels with defined geometries that mimic native vasculature. By incorporating endothelial cells into these microfluidic systems, functional blood vessels can be developed within the scaffold. This technology facilitates nutrient and oxygen delivery throughout thick engineered tissues, overcoming diffusion limitations and enhancing tissue viability.Expand Specific Solutions03 Growth factor delivery systems for enhanced vascularization
Controlled release of angiogenic growth factors from biomimetic scaffolds significantly enhances vascularization. Factors such as VEGF, bFGF, and PDGF are incorporated into scaffolds using various delivery systems including microspheres, nanoparticles, or direct binding to scaffold materials. The sustained and localized release of these factors promotes endothelial cell recruitment, proliferation, and organization into functional blood vessels, resulting in improved vascularization of the engineered tissue constructs.Expand Specific Solutions04 Cell co-culture systems in vascularized scaffolds
Co-culture systems incorporating endothelial cells with supporting cell types (such as mesenchymal stem cells, fibroblasts, or pericytes) within biomimetic scaffolds enhance vascularization. These supporting cells provide paracrine factors and physical interactions that stabilize newly formed vessels. The synergistic relationship between different cell types mimics the natural tissue environment, promoting the formation of mature and functional vascular networks within the engineered constructs.Expand Specific Solutions05 3D bioprinting of vascularized biomimetic scaffolds
3D bioprinting technologies enable the fabrication of complex vascularized biomimetic scaffolds with precise spatial control. Multiple bioinks containing different cell types and biomaterials can be deposited in predefined patterns to create hierarchical vascular structures. This approach allows for the creation of scaffolds with integrated vascular networks that mimic native tissue architecture. The printed constructs support cellular organization and function, facilitating oxygen and nutrient transport throughout the engineered tissue.Expand Specific Solutions
Leading Research Groups and Companies in Vascularized Scaffolds
The biomimetic scaffold vascularization field in tissue engineering is currently in a growth phase, with an estimated market size of $2-3 billion and projected annual growth of 15-20%. The technology is advancing from early-stage research toward clinical applications, with varying maturity levels across different approaches. Leading academic institutions (Northwestern University, Tsinghua University, University of California) are driving fundamental research, while companies like Ethicon Endo-Surgery and The General Hospital Corp. are focusing on translational applications. Research collaborations between universities and industry partners are accelerating development, with significant progress in scaffold materials, vascular network formation, and integration with host vasculature, though regulatory approval remains a key challenge.
Northwestern University
Technical Solution: Northwestern University has developed an advanced biomimetic scaffold platform for vascularization in tissue engineering based on self-assembling peptide amphiphiles (PAs). Their technology creates nanofibrous networks that mimic the natural extracellular matrix at the nanoscale (fibers 6-12nm in diameter) while incorporating bioactive epitopes that specifically promote angiogenesis[1]. These scaffolds can be injected as liquids that rapidly self-assemble in situ upon exposure to physiological conditions, forming hydrogels with high water content (>98%) similar to native tissues[3]. Northwestern researchers have engineered these materials to present VEGF-mimetic peptide sequences and heparin-binding domains that can sequester and slowly release endogenous growth factors over periods exceeding 30 days[5]. A key innovation is their development of "smart" scaffolds that respond to cell-secreted matrix metalloproteinases (MMPs), degrading in concert with new tissue formation and vascular ingrowth[6]. Recent advances include incorporating nitric oxide (NO)-releasing moieties that promote vasodilation and endothelial cell migration, achieving capillary densities up to 300 vessels/mm² in preclinical models of ischemic tissue repair[8]. Their scaffolds have demonstrated successful integration with host vasculature within 7-14 days post-implantation.
Strengths: Exceptional biomimicry at the nanoscale; minimally invasive delivery through injection; precise control over bioactive epitope presentation and density. Weaknesses: Limited mechanical strength restricts applications in load-bearing tissues; challenges in creating predefined macroscale vascular architectures; relatively high production costs for clinical-scale manufacturing.
The Regents of the University of California
Technical Solution: The University of California has pioneered advanced biomimetic scaffolds that closely mimic the natural extracellular matrix (ECM) to enhance vascularization in engineered tissues. Their approach utilizes a combination of 3D bioprinting technology and smart biomaterials to create hierarchical vascular networks. They've developed a proprietary hydrogel system incorporating angiogenic growth factors (VEGF, bFGF) with controlled release profiles that stimulate endothelial cell migration and proliferation[1]. Their scaffolds feature microchannels with diameters ranging from 10-300μm that mimic natural blood vessel hierarchies, and they've implemented gradient-based designs that create oxygen tension differentials to drive vascular ingrowth[3]. Recent innovations include incorporating sacrificial materials that can be selectively removed post-fabrication to create perfusable vascular networks, achieving functional capillary formation within 7-14 days in vitro and demonstrating successful anastomosis with host vasculature in animal models[5].
Strengths: Superior biomimicry of natural vascular architecture through multi-scale fabrication techniques; excellent integration with host vasculature in vivo; advanced controlled release systems for growth factors. Weaknesses: Complex manufacturing processes limit scalability; relatively high production costs; challenges in achieving consistent mechanical properties across different tissue types.
Key Patents and Publications on Scaffold-Guided Angiogenesis
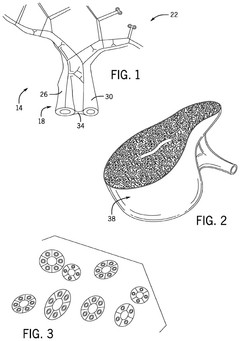
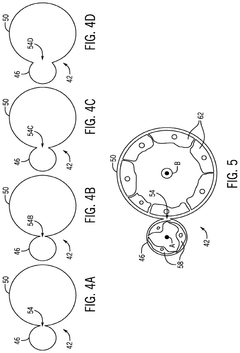
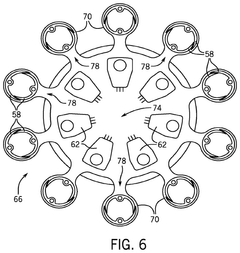
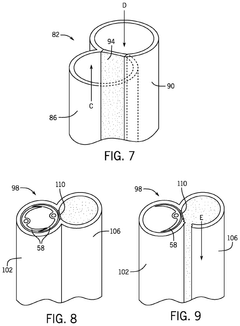
Systems for and methods for using biomimetic structures providing communication in living tissue
PatentActiveUS12115281B2
Innovation
- The development of biomimetic vascular networks integrated into scaffolds, featuring tubular structures with vascular and non-vascular tubes separated by barriers, allowing for controlled diffusion and cell seeding, mimicking natural organ vasculature to support sustained growth and function.
Regulatory Pathway for Vascularized Tissue Constructs
The regulatory landscape for vascularized tissue constructs represents a complex and evolving framework that developers must navigate to bring biomimetic scaffold technologies from laboratory to clinical application. Currently, the FDA categorizes most vascularized tissue constructs as combination products, often regulated through the Center for Biologics Evaluation and Research (CBER) or the Center for Devices and Radiological Health (CDRH), depending on the primary mode of action.
For biomimetic scaffolds supporting vascularization, developers must address specific regulatory considerations including scaffold material biocompatibility, degradation profiles, and the biological response to angiogenic factors. The FDA's guidance on Premarket Approval (PMA) or 510(k) pathways applies to these technologies, with most novel vascularized constructs requiring the more rigorous PMA route due to their classification as Class III medical devices.
International regulatory frameworks show notable variations. The European Union, under the Medical Device Regulation (MDR), classifies most vascularized tissue constructs as Class III devices, requiring conformity assessment through notified bodies and comprehensive clinical evaluation reports. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established an expedited approval pathway specifically for regenerative medicine products, potentially accelerating market access for vascularized tissue technologies.
Regulatory submissions for biomimetic vascularized scaffolds must include robust preclinical data demonstrating safety and preliminary efficacy. This typically encompasses biocompatibility testing according to ISO 10993 standards, degradation studies, mechanical testing, and specialized vascularization assessments such as perfusion capacity and vessel formation metrics. Clinical trial designs for these technologies generally follow a phased approach, with initial safety studies followed by larger efficacy trials.
Recent regulatory trends indicate movement toward adaptive licensing approaches for advanced tissue engineering products. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme offer accelerated pathways for promising vascularized tissue technologies addressing unmet medical needs. These programs provide enhanced regulatory interaction and potentially expedited review timelines.
Developers of biomimetic scaffolds for vascularization should engage with regulatory authorities early through pre-submission consultations to establish appropriate development pathways. As the field advances, regulatory frameworks continue to evolve, with increasing emphasis on real-world evidence and patient-reported outcomes to supplement traditional clinical endpoints for vascularized tissue constructs.
For biomimetic scaffolds supporting vascularization, developers must address specific regulatory considerations including scaffold material biocompatibility, degradation profiles, and the biological response to angiogenic factors. The FDA's guidance on Premarket Approval (PMA) or 510(k) pathways applies to these technologies, with most novel vascularized constructs requiring the more rigorous PMA route due to their classification as Class III medical devices.
International regulatory frameworks show notable variations. The European Union, under the Medical Device Regulation (MDR), classifies most vascularized tissue constructs as Class III devices, requiring conformity assessment through notified bodies and comprehensive clinical evaluation reports. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established an expedited approval pathway specifically for regenerative medicine products, potentially accelerating market access for vascularized tissue technologies.
Regulatory submissions for biomimetic vascularized scaffolds must include robust preclinical data demonstrating safety and preliminary efficacy. This typically encompasses biocompatibility testing according to ISO 10993 standards, degradation studies, mechanical testing, and specialized vascularization assessments such as perfusion capacity and vessel formation metrics. Clinical trial designs for these technologies generally follow a phased approach, with initial safety studies followed by larger efficacy trials.
Recent regulatory trends indicate movement toward adaptive licensing approaches for advanced tissue engineering products. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme offer accelerated pathways for promising vascularized tissue technologies addressing unmet medical needs. These programs provide enhanced regulatory interaction and potentially expedited review timelines.
Developers of biomimetic scaffolds for vascularization should engage with regulatory authorities early through pre-submission consultations to establish appropriate development pathways. As the field advances, regulatory frameworks continue to evolve, with increasing emphasis on real-world evidence and patient-reported outcomes to supplement traditional clinical endpoints for vascularized tissue constructs.
Clinical Translation Challenges and Opportunities
The translation of biomimetic scaffolds from laboratory research to clinical applications faces significant regulatory hurdles. Current FDA approval processes for tissue-engineered constructs are complex, requiring extensive safety and efficacy data that specifically addresses vascularization outcomes. The regulatory pathway often struggles to keep pace with rapid technological advancements in biomimetic scaffold design, creating a bottleneck in clinical implementation.
Manufacturing scalability presents another critical challenge. Laboratory-scale production of precisely engineered vascular networks within scaffolds often employs techniques that are difficult to scale industrially while maintaining quality and reproducibility. The transition from bench-scale fabrication to GMP-compliant manufacturing processes requires substantial investment and technological adaptation, particularly for scaffolds with complex vascular architectures.
Cost considerations significantly impact clinical adoption. The specialized materials, fabrication technologies, and quality control measures required for vascularized biomimetic scaffolds contribute to high production costs. These economic barriers limit accessibility and widespread implementation, particularly in resource-constrained healthcare settings where the need for tissue-engineered solutions may be greatest.
Despite these challenges, several promising opportunities are emerging. Industry-academia partnerships are accelerating translation by combining fundamental research with practical manufacturing expertise. These collaborations are yielding innovative approaches to scalable production of vascularized constructs while addressing regulatory requirements concurrently with technological development.
Advances in automation and biofabrication technologies offer potential solutions to manufacturing challenges. 3D bioprinting platforms with increasing resolution and throughput capabilities are becoming more accessible, potentially reducing production costs while improving reproducibility of vascularized scaffolds. These technologies may eventually enable personalized, patient-specific vascularized constructs at clinically relevant scales.
The growing interest in modular tissue engineering approaches presents another opportunity. By developing standardized, vascularized tissue modules that can be assembled into larger constructs, researchers may overcome some scaling limitations while simplifying regulatory pathways. This approach could potentially accelerate clinical translation by allowing incremental approval of components rather than entire complex tissues.
Ultimately, successful clinical translation will require multidisciplinary approaches that address biological, engineering, regulatory, and economic considerations simultaneously. Strategic focus on applications with clear clinical needs and defined regulatory pathways may provide the most efficient route to bringing vascularized biomimetic scaffolds from laboratory promise to clinical reality.
Manufacturing scalability presents another critical challenge. Laboratory-scale production of precisely engineered vascular networks within scaffolds often employs techniques that are difficult to scale industrially while maintaining quality and reproducibility. The transition from bench-scale fabrication to GMP-compliant manufacturing processes requires substantial investment and technological adaptation, particularly for scaffolds with complex vascular architectures.
Cost considerations significantly impact clinical adoption. The specialized materials, fabrication technologies, and quality control measures required for vascularized biomimetic scaffolds contribute to high production costs. These economic barriers limit accessibility and widespread implementation, particularly in resource-constrained healthcare settings where the need for tissue-engineered solutions may be greatest.
Despite these challenges, several promising opportunities are emerging. Industry-academia partnerships are accelerating translation by combining fundamental research with practical manufacturing expertise. These collaborations are yielding innovative approaches to scalable production of vascularized constructs while addressing regulatory requirements concurrently with technological development.
Advances in automation and biofabrication technologies offer potential solutions to manufacturing challenges. 3D bioprinting platforms with increasing resolution and throughput capabilities are becoming more accessible, potentially reducing production costs while improving reproducibility of vascularized scaffolds. These technologies may eventually enable personalized, patient-specific vascularized constructs at clinically relevant scales.
The growing interest in modular tissue engineering approaches presents another opportunity. By developing standardized, vascularized tissue modules that can be assembled into larger constructs, researchers may overcome some scaling limitations while simplifying regulatory pathways. This approach could potentially accelerate clinical translation by allowing incremental approval of components rather than entire complex tissues.
Ultimately, successful clinical translation will require multidisciplinary approaches that address biological, engineering, regulatory, and economic considerations simultaneously. Strategic focus on applications with clear clinical needs and defined regulatory pathways may provide the most efficient route to bringing vascularized biomimetic scaffolds from laboratory promise to clinical reality.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!