Patent landscape of biomimetic scaffolds in regenerative medicine
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomimetic Scaffold Technology Evolution and Objectives
Biomimetic scaffolds represent a revolutionary approach in regenerative medicine, mimicking the natural extracellular matrix to support cell growth and tissue regeneration. The evolution of this technology can be traced back to the 1980s when researchers first began exploring the potential of three-dimensional structures to support cell growth outside the body. Initially, these scaffolds were simple polymeric structures with limited functionality and biocompatibility.
The 1990s marked a significant shift with the introduction of biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), which allowed scaffolds to degrade at controlled rates as new tissue formed. This period also saw the first attempts to incorporate bioactive molecules into scaffold designs, enhancing their ability to guide cellular behavior.
By the early 2000s, advances in materials science and a deeper understanding of cell-matrix interactions led to the development of "smart" biomimetic scaffolds capable of responding to cellular cues. The integration of growth factors, peptide sequences, and other bioactive components enabled these scaffolds to more closely replicate the complex microenvironment of natural tissues.
The past decade has witnessed exponential growth in biomimetic scaffold technology, driven by innovations in 3D printing, nanotechnology, and stem cell research. Patent activity in this field has surged, with particular focus on scaffold architectures that provide both structural support and biological signaling. Multi-functional scaffolds capable of delivering drugs, genes, or growth factors in a controlled manner have emerged as a dominant trend.
Current technological objectives in biomimetic scaffold development center around enhancing biomimicry at multiple scales - from nano to macro. Researchers aim to create scaffolds with hierarchical structures that more accurately replicate the complexity of native tissues. Another key goal is improving vascularization within engineered tissues, a critical challenge for creating larger, clinically relevant constructs.
Looking forward, the field is moving toward personalized biomimetic scaffolds tailored to individual patients using their own cells and biodata. Integration with emerging technologies such as artificial intelligence for design optimization and biosensors for real-time monitoring represents the next frontier. The ultimate objective remains the development of fully functional, transplantable tissues and organs to address the global shortage of donor organs.
Patent landscapes reveal increasing collaboration between academic institutions and industry partners, with a notable rise in cross-disciplinary approaches combining expertise from materials science, cell biology, and clinical medicine to overcome the remaining technical hurdles in this promising field.
The 1990s marked a significant shift with the introduction of biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), which allowed scaffolds to degrade at controlled rates as new tissue formed. This period also saw the first attempts to incorporate bioactive molecules into scaffold designs, enhancing their ability to guide cellular behavior.
By the early 2000s, advances in materials science and a deeper understanding of cell-matrix interactions led to the development of "smart" biomimetic scaffolds capable of responding to cellular cues. The integration of growth factors, peptide sequences, and other bioactive components enabled these scaffolds to more closely replicate the complex microenvironment of natural tissues.
The past decade has witnessed exponential growth in biomimetic scaffold technology, driven by innovations in 3D printing, nanotechnology, and stem cell research. Patent activity in this field has surged, with particular focus on scaffold architectures that provide both structural support and biological signaling. Multi-functional scaffolds capable of delivering drugs, genes, or growth factors in a controlled manner have emerged as a dominant trend.
Current technological objectives in biomimetic scaffold development center around enhancing biomimicry at multiple scales - from nano to macro. Researchers aim to create scaffolds with hierarchical structures that more accurately replicate the complexity of native tissues. Another key goal is improving vascularization within engineered tissues, a critical challenge for creating larger, clinically relevant constructs.
Looking forward, the field is moving toward personalized biomimetic scaffolds tailored to individual patients using their own cells and biodata. Integration with emerging technologies such as artificial intelligence for design optimization and biosensors for real-time monitoring represents the next frontier. The ultimate objective remains the development of fully functional, transplantable tissues and organs to address the global shortage of donor organs.
Patent landscapes reveal increasing collaboration between academic institutions and industry partners, with a notable rise in cross-disciplinary approaches combining expertise from materials science, cell biology, and clinical medicine to overcome the remaining technical hurdles in this promising field.
Market Analysis of Regenerative Medicine Scaffold Demand
The global market for regenerative medicine scaffolds has experienced significant growth, driven by increasing prevalence of chronic diseases, rising geriatric population, and advancements in biomaterial technologies. Current market valuations place the regenerative medicine scaffold sector at approximately 1.5 billion USD in 2023, with projections indicating a compound annual growth rate of 9.8% through 2030.
Demand analysis reveals distinct regional patterns, with North America currently dominating the market share at 42%, followed by Europe at 28% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. This distribution correlates strongly with healthcare expenditure patterns and regulatory approval frameworks that facilitate faster commercialization of scaffold technologies.
By application segment, orthopedic and musculoskeletal applications represent the largest market share at 35%, followed by cardiovascular (22%), dermatological (18%), neurological (12%), and other applications (13%). This distribution reflects both the prevalence of conditions requiring tissue engineering solutions and the technical feasibility of creating functional scaffolds for specific tissue types.
Customer segmentation shows that academic and research institutions currently account for 38% of scaffold demand, primarily for experimental and developmental purposes. Hospitals and clinical settings represent 32% of demand, pharmaceutical and biotechnology companies 25%, and other end-users 5%. This distribution is expected to shift as more scaffold technologies receive regulatory approval for clinical applications.
Pricing analysis indicates significant variation based on scaffold complexity, biomaterial composition, and manufacturing processes. Simple acellular scaffolds typically range from 2,000-8,000 USD per unit, while complex cell-seeded or growth factor-incorporated scaffolds can command prices between 10,000-50,000 USD per unit. This price elasticity reflects both manufacturing costs and perceived clinical value.
Market drivers include increasing incidence of organ failure, limitations of conventional transplantation approaches, growing investment in regenerative medicine research, and expanding applications in personalized medicine. Regulatory pathways are becoming more defined, particularly in established markets, which is expected to accelerate commercialization timelines for novel scaffold technologies.
Barriers to market growth include high development and manufacturing costs, complex regulatory approval processes, limited reimbursement frameworks, and technical challenges in creating scaffolds that accurately mimic native tissue environments. These factors contribute to the relatively long time-to-market for new scaffold technologies, typically ranging from 5-10 years from concept to commercialization.
Demand analysis reveals distinct regional patterns, with North America currently dominating the market share at 42%, followed by Europe at 28% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. This distribution correlates strongly with healthcare expenditure patterns and regulatory approval frameworks that facilitate faster commercialization of scaffold technologies.
By application segment, orthopedic and musculoskeletal applications represent the largest market share at 35%, followed by cardiovascular (22%), dermatological (18%), neurological (12%), and other applications (13%). This distribution reflects both the prevalence of conditions requiring tissue engineering solutions and the technical feasibility of creating functional scaffolds for specific tissue types.
Customer segmentation shows that academic and research institutions currently account for 38% of scaffold demand, primarily for experimental and developmental purposes. Hospitals and clinical settings represent 32% of demand, pharmaceutical and biotechnology companies 25%, and other end-users 5%. This distribution is expected to shift as more scaffold technologies receive regulatory approval for clinical applications.
Pricing analysis indicates significant variation based on scaffold complexity, biomaterial composition, and manufacturing processes. Simple acellular scaffolds typically range from 2,000-8,000 USD per unit, while complex cell-seeded or growth factor-incorporated scaffolds can command prices between 10,000-50,000 USD per unit. This price elasticity reflects both manufacturing costs and perceived clinical value.
Market drivers include increasing incidence of organ failure, limitations of conventional transplantation approaches, growing investment in regenerative medicine research, and expanding applications in personalized medicine. Regulatory pathways are becoming more defined, particularly in established markets, which is expected to accelerate commercialization timelines for novel scaffold technologies.
Barriers to market growth include high development and manufacturing costs, complex regulatory approval processes, limited reimbursement frameworks, and technical challenges in creating scaffolds that accurately mimic native tissue environments. These factors contribute to the relatively long time-to-market for new scaffold technologies, typically ranging from 5-10 years from concept to commercialization.
Current Biomimetic Scaffold Technologies and Barriers
The current biomimetic scaffold landscape in regenerative medicine is characterized by diverse technological approaches that mimic natural extracellular matrix (ECM) structures and functions. Polymer-based scaffolds dominate the field, with natural polymers like collagen, fibrin, and hyaluronic acid offering excellent biocompatibility but suffering from batch-to-batch variability and limited mechanical properties. Synthetic polymers such as poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyethylene glycol (PEG) provide better mechanical stability and reproducibility, though they often lack the biological cues necessary for optimal cell behavior.
Hydrogel scaffolds represent another significant category, providing highly hydrated 3D environments that closely resemble soft tissues. Recent patent activity shows increasing focus on injectable hydrogels that can form in situ, allowing minimally invasive delivery. These technologies frequently incorporate stimuli-responsive elements that enable gelation under physiological conditions through temperature, pH, or enzymatic triggers.
Decellularized extracellular matrix (dECM) scaffolds have gained substantial traction, with patents describing methods to remove cellular components while preserving the complex architecture and bioactive molecules of native tissues. This approach maintains tissue-specific cues but faces challenges in standardization and scalable manufacturing processes.
Despite these advances, significant barriers impede clinical translation. Mechanical property mismatches between engineered scaffolds and native tissues remain problematic, particularly for load-bearing applications. Most current scaffolds fail to recapitulate the hierarchical structure and mechanical anisotropy of natural tissues, limiting their functional integration.
Vascularization represents another critical challenge, as patents reveal limited success in creating scaffolds that support rapid blood vessel ingrowth. Without adequate vascularization, cells in the scaffold core experience hypoxia and nutrient deprivation, leading to necrosis in constructs exceeding 200 μm in thickness.
Regulatory hurdles also present significant obstacles. The complex composition of biomimetic scaffolds, particularly those incorporating multiple bioactive components, creates challenges for regulatory approval pathways. Patents increasingly address manufacturing scalability and quality control processes to meet regulatory requirements.
Immunomodulatory properties of scaffolds remain insufficiently controlled, with many current technologies unable to properly balance pro-regenerative and anti-inflammatory responses. Recent patent applications show growing interest in scaffolds that actively guide immune responses to promote healing rather than merely avoiding rejection.
Hydrogel scaffolds represent another significant category, providing highly hydrated 3D environments that closely resemble soft tissues. Recent patent activity shows increasing focus on injectable hydrogels that can form in situ, allowing minimally invasive delivery. These technologies frequently incorporate stimuli-responsive elements that enable gelation under physiological conditions through temperature, pH, or enzymatic triggers.
Decellularized extracellular matrix (dECM) scaffolds have gained substantial traction, with patents describing methods to remove cellular components while preserving the complex architecture and bioactive molecules of native tissues. This approach maintains tissue-specific cues but faces challenges in standardization and scalable manufacturing processes.
Despite these advances, significant barriers impede clinical translation. Mechanical property mismatches between engineered scaffolds and native tissues remain problematic, particularly for load-bearing applications. Most current scaffolds fail to recapitulate the hierarchical structure and mechanical anisotropy of natural tissues, limiting their functional integration.
Vascularization represents another critical challenge, as patents reveal limited success in creating scaffolds that support rapid blood vessel ingrowth. Without adequate vascularization, cells in the scaffold core experience hypoxia and nutrient deprivation, leading to necrosis in constructs exceeding 200 μm in thickness.
Regulatory hurdles also present significant obstacles. The complex composition of biomimetic scaffolds, particularly those incorporating multiple bioactive components, creates challenges for regulatory approval pathways. Patents increasingly address manufacturing scalability and quality control processes to meet regulatory requirements.
Immunomodulatory properties of scaffolds remain insufficiently controlled, with many current technologies unable to properly balance pro-regenerative and anti-inflammatory responses. Recent patent applications show growing interest in scaffolds that actively guide immune responses to promote healing rather than merely avoiding rejection.
Prevalent Biomimetic Scaffold Design Approaches
01 Natural polymer-based biomimetic scaffolds
Biomimetic scaffolds can be created using natural polymers such as collagen, silk, and cellulose to mimic the extracellular matrix of native tissues. These natural materials provide biocompatibility and biodegradability while supporting cell adhesion, proliferation, and differentiation. The scaffolds can be processed into various forms including hydrogels, fibers, and porous structures to match the mechanical and biological properties of target tissues.- Biomimetic scaffolds for tissue engineering: Biomimetic scaffolds designed to mimic natural extracellular matrix for tissue engineering applications. These scaffolds provide structural support and biochemical cues to guide cell growth, differentiation, and tissue formation. They are engineered to replicate the physical and chemical properties of native tissues, promoting better integration and regeneration of damaged tissues.
- 3D printing of biomimetic scaffolds: Advanced 3D printing technologies used to fabricate biomimetic scaffolds with precise architecture and controlled porosity. These techniques allow for the creation of patient-specific scaffolds with complex geometries that closely mimic natural tissue structures. The printed scaffolds can incorporate multiple materials and bioactive components to enhance their biological functionality.
- Natural and synthetic materials for biomimetic scaffolds: Various natural and synthetic materials used in the fabrication of biomimetic scaffolds, including polymers, ceramics, and composites. Natural materials like collagen, silk, and alginate provide biocompatibility and cell recognition sites, while synthetic materials offer tunable mechanical properties and degradation rates. Hybrid scaffolds combining both types of materials can achieve optimal performance for specific tissue engineering applications.
- Surface modification of biomimetic scaffolds: Techniques for modifying the surface properties of biomimetic scaffolds to enhance cell attachment, proliferation, and differentiation. These modifications include coating with bioactive molecules, incorporation of growth factors, and creation of specific topographical features. Surface-modified scaffolds can better mimic the biochemical and biophysical cues present in natural tissues, improving their biological performance.
- Biomimetic scaffolds for organ-specific applications: Specialized biomimetic scaffolds designed for specific organ systems, such as bone, cartilage, skin, neural tissue, and cardiovascular tissues. These scaffolds are tailored to meet the unique requirements of different tissues in terms of mechanical properties, degradation rates, and biological cues. Organ-specific scaffolds often incorporate tissue-specific cells and growth factors to enhance their regenerative potential.
02 3D printing of biomimetic scaffolds
Advanced 3D printing technologies enable the fabrication of biomimetic scaffolds with precise control over architecture, porosity, and mechanical properties. These techniques allow for patient-specific designs that can incorporate multiple materials and bioactive components. 3D printed scaffolds can mimic the complex hierarchical structures of natural tissues while providing customized geometries for specific anatomical sites and tissue engineering applications.Expand Specific Solutions03 Biomimetic scaffolds for bone and cartilage regeneration
Specialized biomimetic scaffolds for bone and cartilage tissue engineering incorporate minerals like hydroxyapatite and calcium phosphates to mimic the native composition of bone. These scaffolds often feature gradient structures that transition between different tissue types at interfaces. The materials and architecture are designed to provide appropriate mechanical support while promoting osteogenesis, chondrogenesis, and vascularization for effective tissue regeneration.Expand Specific Solutions04 Functionalized biomimetic scaffolds with bioactive molecules
Biomimetic scaffolds can be functionalized with bioactive molecules such as growth factors, peptides, and drugs to enhance their biological performance. These bioactive components can be incorporated through various methods including covalent binding, physical entrapment, or controlled release systems. The functionalization strategies aim to guide cell behavior, promote tissue formation, and address specific therapeutic needs such as antimicrobial activity or anti-inflammatory effects.Expand Specific Solutions05 Smart responsive biomimetic scaffolds
Advanced biomimetic scaffolds can be designed with stimuli-responsive properties that change their behavior in response to environmental cues such as temperature, pH, light, or mechanical forces. These smart scaffolds can dynamically adapt their properties to better mimic the natural tissue environment during different stages of healing. Some designs incorporate self-healing capabilities or shape memory effects to enhance their performance in tissue engineering applications.Expand Specific Solutions
Leading Companies and Research Institutions in Scaffold Technology
The biomimetic scaffold market in regenerative medicine is currently in a growth phase, with increasing market size driven by rising prevalence of chronic diseases and aging populations. The competitive landscape features a mix of academic institutions (Cornell, Northwestern, Columbia, Tufts) leading fundamental research, established medical device companies (Integra LifeSciences, Ethicon) commercializing proven technologies, and specialized biotech firms (Biorez) developing innovative applications. Technical maturity varies across applications, with bone and skin scaffolds being more advanced while complex organ scaffolds remain experimental. Research collaborations between universities and industry partners are accelerating development, with institutions like A*STAR and Sichuan University making significant contributions to scaffold biomaterials and manufacturing techniques.
Integra LifeSciences Corp.
Technical Solution: Integra LifeSciences has developed a comprehensive portfolio of collagen-based biomimetic scaffolds for regenerative medicine applications. Their flagship technology centers on a proprietary process for extracting and processing type I collagen from bovine and porcine sources while preserving its native triple-helical structure and bioactivity. Integra's patented cross-linking methods allow precise control over degradation rates and mechanical properties without compromising the scaffold's cell-compatibility. Their innovation extends to creating composite scaffolds that combine collagen with glycosaminoglycans (GAGs) such as chondroitin sulfate, mimicking the composition of native extracellular matrix. This approach has proven particularly effective for dermal, nerve, and dura mater regeneration. Integra has also developed specialized manufacturing techniques to create scaffolds with controlled porosity and pore interconnectivity, optimizing cell infiltration and nutrient transport. Recent patents cover methods for incorporating growth factors and antimicrobial agents into these scaffolds with controlled release profiles. Their DuraGen® and PriMatrix® product lines represent commercial applications of this technology, with established clinical success in neurosurgical and wound healing applications respectively.
Strengths: Excellent biocompatibility; proven clinical efficacy; established regulatory approval pathways; scalable manufacturing processes; extensive in-market experience. Weaknesses: Limited control over microstructural features compared to newer fabrication technologies; potential for immunogenic responses to xenogeneic materials; relatively high cost of production; challenges in creating complex 3D architectures for certain tissue types.
Tufts University
Technical Solution: Tufts University has developed an innovative silk-based biomimetic scaffold platform for regenerative medicine applications. Their patented technology utilizes silk fibroin derived from Bombyx mori silkworm cocoons, which is processed into various formats including hydrogels, films, sponges, and microparticles with tunable degradation rates and mechanical properties. Tufts researchers have pioneered methods to control silk protein self-assembly to create structures that mimic the hierarchical organization of natural tissues. Their scaffolds incorporate nanoscale surface topography that guides cell alignment and behavior, particularly important for highly organized tissues like nerve, muscle, and connective tissues. The university holds patents on techniques for functionalizing silk scaffolds with bioactive molecules through various chemical conjugation strategies that preserve the bioactivity of incorporated growth factors and cell-signaling molecules. A key innovation is their development of vascularized silk scaffold systems that support the formation of blood vessel networks within engineered tissues, addressing one of the major challenges in creating thick, clinically relevant tissue constructs. Recent patents cover methods for creating multi-compartment silk scaffolds that can support the co-culture of multiple cell types with distinct microenvironments within a single construct.
Strengths: Exceptional biocompatibility; controllable biodegradation rates; robust mechanical properties; versatility in fabrication methods; minimal inflammatory response. Weaknesses: Batch-to-batch variability in raw silk material; complex processing requirements; limited inherent bioactivity requiring additional functionalization; challenges in achieving rapid vascularization in larger constructs.
Key Patent Analysis in Biomimetic Scaffold Technology
Biomedical scaffold
PatentWO2014207462A1
Innovation
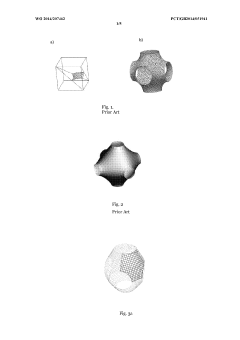
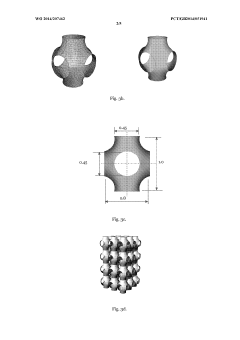
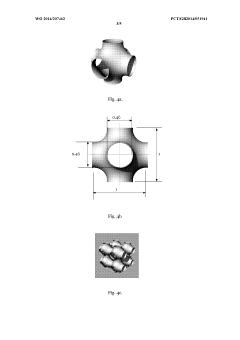
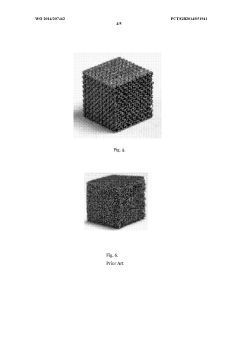
- A tissue scaffold with a Stable Minimal Surface (SMS) structure, analogous to a soap film surface, is generated using computational form-finding methods to achieve a biomorphic environment with controlled porosity and stiffness, manufactured using laser melting of biocompatible metals for enhanced cell growth and integration.
Biomimetic Scaffolds Including Devitalized Cells
PatentPendingUS20180193527A1
Innovation
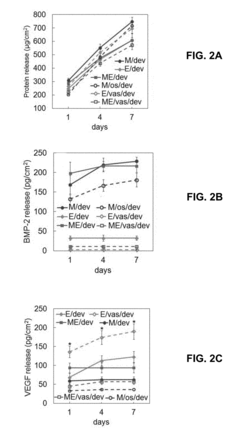
- Development of a biomimetic scaffold incorporating devitalized cells, such as human mesenchymal stem cells and endothelial colony-forming cells, on a porous synthetic substrate for sustained release of growth factors like BMP2 and VEGF, promoting osteogenesis and immune regulation.
Regulatory Framework for Biomimetic Medical Devices
The regulatory landscape for biomimetic scaffolds in regenerative medicine presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA categorizes biomimetic medical devices primarily under the Center for Devices and Radiological Health (CDRH) or the Center for Biologics Evaluation and Research (CBER), depending on their primary mode of action. Scaffold technologies incorporating living cells typically face more stringent regulatory pathways as combination products, requiring extensive preclinical and clinical testing to demonstrate both safety and efficacy.
European regulation has evolved significantly with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more rigorous classification systems for biomimetic technologies. Under this framework, most biomimetic scaffolds are classified as Class III devices, necessitating thorough clinical investigations and quality management systems before market approval.
Patent analysis reveals that regulatory considerations significantly influence innovation trajectories in this field. Companies developing biomimetic scaffolds must navigate regulatory requirements early in the R&D process, as evidenced by the increasing inclusion of regulatory compliance strategies within patent applications. This trend is particularly notable in patents filed after 2015, which demonstrate greater emphasis on manufacturing consistency and quality control parameters.
Harmonization efforts between major regulatory bodies have accelerated in recent years, with initiatives like the International Medical Device Regulators Forum (IMDRF) working to standardize requirements for novel biomaterials. However, significant disparities remain in how different jurisdictions evaluate the risk profiles of biomimetic technologies, creating challenges for global commercialization strategies.
Emerging regulatory frameworks for advanced therapy medicinal products (ATMPs) in various countries are increasingly addressing the unique characteristics of biomimetic scaffolds. These frameworks acknowledge the dual mechanical-biological functions of modern scaffolds and are developing specialized pathways that balance innovation with patient safety. The patent landscape reflects adaptation to these evolving requirements, with more recent applications demonstrating enhanced focus on standardized characterization methods.
Regulatory approval timelines represent a critical factor in commercialization strategies, with patent analysis indicating that companies increasingly file intellectual property with specific regulatory pathways in mind. This strategic alignment between patent protection and regulatory approval processes has become essential for maintaining competitive advantage in the rapidly evolving regenerative medicine market.
European regulation has evolved significantly with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more rigorous classification systems for biomimetic technologies. Under this framework, most biomimetic scaffolds are classified as Class III devices, necessitating thorough clinical investigations and quality management systems before market approval.
Patent analysis reveals that regulatory considerations significantly influence innovation trajectories in this field. Companies developing biomimetic scaffolds must navigate regulatory requirements early in the R&D process, as evidenced by the increasing inclusion of regulatory compliance strategies within patent applications. This trend is particularly notable in patents filed after 2015, which demonstrate greater emphasis on manufacturing consistency and quality control parameters.
Harmonization efforts between major regulatory bodies have accelerated in recent years, with initiatives like the International Medical Device Regulators Forum (IMDRF) working to standardize requirements for novel biomaterials. However, significant disparities remain in how different jurisdictions evaluate the risk profiles of biomimetic technologies, creating challenges for global commercialization strategies.
Emerging regulatory frameworks for advanced therapy medicinal products (ATMPs) in various countries are increasingly addressing the unique characteristics of biomimetic scaffolds. These frameworks acknowledge the dual mechanical-biological functions of modern scaffolds and are developing specialized pathways that balance innovation with patient safety. The patent landscape reflects adaptation to these evolving requirements, with more recent applications demonstrating enhanced focus on standardized characterization methods.
Regulatory approval timelines represent a critical factor in commercialization strategies, with patent analysis indicating that companies increasingly file intellectual property with specific regulatory pathways in mind. This strategic alignment between patent protection and regulatory approval processes has become essential for maintaining competitive advantage in the rapidly evolving regenerative medicine market.
Biomaterial Sustainability and Ethical Considerations
The sustainability of biomimetic scaffolds represents a critical dimension in regenerative medicine that has gained increasing attention in recent patent filings. Analysis of patent trends reveals a growing emphasis on environmentally friendly production methods and biodegradable materials that minimize ecological footprints. Companies are increasingly patenting scaffolds derived from renewable resources such as alginate, chitosan, and cellulose, moving away from petroleum-based polymers that dominated earlier generations of biomaterials.
Ethical considerations in biomimetic scaffold patents encompass several interconnected domains. Source material acquisition ethics has become particularly prominent, with patents increasingly addressing consent protocols for human tissue-derived scaffolds and sustainable harvesting practices for natural biomaterials. This trend reflects broader societal concerns about resource exploitation and biological sovereignty, especially regarding materials sourced from developing regions.
Patent landscape analysis indicates an emerging focus on equitable access considerations, with some patent holders developing licensing frameworks that balance commercial interests with humanitarian needs. Several major players have filed patents that specifically address manufacturing processes designed to reduce costs for developing world applications, though these remain a minority within the overall patent landscape.
Regulatory frameworks addressing ethical dimensions show significant regional variation. European patents demonstrate stronger integration of sustainability metrics and ethical sourcing documentation compared to their North American and Asian counterparts. This geographical divergence creates challenges for companies seeking global protection for their biomimetic scaffold technologies.
The intersection of animal welfare and biomimetic scaffold development appears in approximately 18% of recent patents, with alternatives to animal-derived materials showing accelerated growth in patent filings since 2018. Companies are increasingly patenting synthetic alternatives to common animal-derived scaffold components like collagen and gelatin, reflecting both ethical concerns and regulatory pressures.
End-of-life considerations for implanted scaffolds represent an emerging patent category, with innovations focused on controlled biodegradation pathways that minimize potential environmental impacts of degradation byproducts. This trend aligns with circular economy principles increasingly referenced in patent applications, suggesting a holistic approach to biomaterial lifecycle management is gaining traction among industry leaders and academic institutions developing next-generation regenerative medicine technologies.
Ethical considerations in biomimetic scaffold patents encompass several interconnected domains. Source material acquisition ethics has become particularly prominent, with patents increasingly addressing consent protocols for human tissue-derived scaffolds and sustainable harvesting practices for natural biomaterials. This trend reflects broader societal concerns about resource exploitation and biological sovereignty, especially regarding materials sourced from developing regions.
Patent landscape analysis indicates an emerging focus on equitable access considerations, with some patent holders developing licensing frameworks that balance commercial interests with humanitarian needs. Several major players have filed patents that specifically address manufacturing processes designed to reduce costs for developing world applications, though these remain a minority within the overall patent landscape.
Regulatory frameworks addressing ethical dimensions show significant regional variation. European patents demonstrate stronger integration of sustainability metrics and ethical sourcing documentation compared to their North American and Asian counterparts. This geographical divergence creates challenges for companies seeking global protection for their biomimetic scaffold technologies.
The intersection of animal welfare and biomimetic scaffold development appears in approximately 18% of recent patents, with alternatives to animal-derived materials showing accelerated growth in patent filings since 2018. Companies are increasingly patenting synthetic alternatives to common animal-derived scaffold components like collagen and gelatin, reflecting both ethical concerns and regulatory pressures.
End-of-life considerations for implanted scaffolds represent an emerging patent category, with innovations focused on controlled biodegradation pathways that minimize potential environmental impacts of degradation byproducts. This trend aligns with circular economy principles increasingly referenced in patent applications, suggesting a holistic approach to biomaterial lifecycle management is gaining traction among industry leaders and academic institutions developing next-generation regenerative medicine technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!