Hirudoid’s Advancements in Recovery Science
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hirudoid Background and Objectives
Hirudoid, a topical heparin-based gel, has emerged as a significant advancement in recovery science, particularly in the field of sports medicine and post-surgical care. The development of Hirudoid can be traced back to the early 1950s when researchers began exploring the potential of heparin as a topical treatment for various conditions. Over the decades, the formulation has been refined and optimized to enhance its efficacy and safety profile.
The primary objective of Hirudoid's development has been to provide a non-invasive solution for managing soft tissue injuries, bruising, and post-operative recovery. By leveraging the anti-inflammatory and anti-coagulant properties of heparin, Hirudoid aims to accelerate the healing process and reduce recovery time for athletes and patients alike. This aligns with the growing demand for more effective and less invasive treatment options in sports medicine and post-surgical care.
As the field of recovery science has evolved, so too has the focus on developing products that can be easily applied and absorbed by the body. Hirudoid's gel formulation addresses this need by providing a convenient and efficient delivery method for heparin. The technology behind Hirudoid has continually been refined to improve its absorption rate and overall effectiveness, making it a staple in many medical and athletic settings.
The ongoing research and development of Hirudoid reflect broader trends in recovery science, including the emphasis on personalized medicine and the integration of advanced materials science. Scientists are exploring ways to further enhance the product's efficacy by incorporating complementary ingredients and optimizing the gel's molecular structure to maximize heparin delivery to affected tissues.
Looking ahead, the future development of Hirudoid and similar products in recovery science is likely to focus on several key areas. These include improving the product's longevity and sustained release capabilities, enhancing its compatibility with other treatment modalities, and expanding its applications to a wider range of medical conditions. Additionally, there is growing interest in developing formulations that can be tailored to individual patient needs, potentially incorporating elements of genetic profiling and biomarker analysis.
The evolution of Hirudoid serves as a prime example of how continuous innovation in recovery science can lead to significant improvements in patient care and athletic performance. As research in this field progresses, we can expect to see further advancements that build upon the foundation laid by products like Hirudoid, ultimately leading to more effective, personalized, and accessible recovery solutions for a diverse range of applications.
The primary objective of Hirudoid's development has been to provide a non-invasive solution for managing soft tissue injuries, bruising, and post-operative recovery. By leveraging the anti-inflammatory and anti-coagulant properties of heparin, Hirudoid aims to accelerate the healing process and reduce recovery time for athletes and patients alike. This aligns with the growing demand for more effective and less invasive treatment options in sports medicine and post-surgical care.
As the field of recovery science has evolved, so too has the focus on developing products that can be easily applied and absorbed by the body. Hirudoid's gel formulation addresses this need by providing a convenient and efficient delivery method for heparin. The technology behind Hirudoid has continually been refined to improve its absorption rate and overall effectiveness, making it a staple in many medical and athletic settings.
The ongoing research and development of Hirudoid reflect broader trends in recovery science, including the emphasis on personalized medicine and the integration of advanced materials science. Scientists are exploring ways to further enhance the product's efficacy by incorporating complementary ingredients and optimizing the gel's molecular structure to maximize heparin delivery to affected tissues.
Looking ahead, the future development of Hirudoid and similar products in recovery science is likely to focus on several key areas. These include improving the product's longevity and sustained release capabilities, enhancing its compatibility with other treatment modalities, and expanding its applications to a wider range of medical conditions. Additionally, there is growing interest in developing formulations that can be tailored to individual patient needs, potentially incorporating elements of genetic profiling and biomarker analysis.
The evolution of Hirudoid serves as a prime example of how continuous innovation in recovery science can lead to significant improvements in patient care and athletic performance. As research in this field progresses, we can expect to see further advancements that build upon the foundation laid by products like Hirudoid, ultimately leading to more effective, personalized, and accessible recovery solutions for a diverse range of applications.
Market Analysis for Recovery Products
The recovery products market has experienced significant growth in recent years, driven by increasing awareness of health and wellness, rising sports participation, and an aging population seeking improved recovery solutions. Hirudoid, a key player in this market, has positioned itself at the forefront of recovery science advancements.
The global recovery products market is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to exceed 5% over the next five years. This growth is fueled by a combination of factors, including the rising prevalence of sports-related injuries, the growing popularity of high-intensity workouts, and the increasing adoption of recovery techniques among professional athletes and fitness enthusiasts alike.
Hirudoid's products, particularly those focused on topical recovery solutions, have gained traction in both the professional sports and consumer markets. The company's innovative formulations, which leverage advanced ingredients and delivery systems, have resonated with consumers seeking effective recovery options. This has contributed to Hirudoid's market share expansion and brand recognition within the recovery products sector.
The recovery products market can be segmented into various categories, including topical treatments, compression garments, nutrition supplements, and recovery equipment. Hirudoid's primary focus on topical solutions has allowed the company to capitalize on the growing demand for non-invasive, easy-to-use recovery products. This segment of the market is expected to show robust growth, driven by consumer preference for convenient and targeted recovery options.
Geographically, North America and Europe currently dominate the recovery products market, accounting for a significant portion of global sales. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential, presenting opportunities for companies like Hirudoid to expand their global footprint. The increasing disposable income and growing health consciousness in these regions are key factors driving market expansion.
Consumer trends indicate a shift towards more holistic recovery approaches, combining multiple product types and techniques. This trend presents both opportunities and challenges for Hirudoid, as it may need to diversify its product portfolio or form strategic partnerships to offer comprehensive recovery solutions.
The competitive landscape of the recovery products market is characterized by a mix of established pharmaceutical companies, sports equipment manufacturers, and specialized recovery brands. Hirudoid's focus on scientific advancements and product efficacy has helped differentiate its offerings in a crowded marketplace. However, the company faces competition from both large multinational corporations with significant R&D budgets and agile startups leveraging new technologies.
In conclusion, the market analysis for recovery products reveals a promising landscape for Hirudoid's continued growth and innovation. The company's advancements in recovery science position it well to capitalize on the expanding market opportunities, particularly in the topical solutions segment. To maintain its competitive edge, Hirudoid should continue to invest in research and development, explore new market segments, and adapt to evolving consumer preferences in the dynamic recovery products industry.
The global recovery products market is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to exceed 5% over the next five years. This growth is fueled by a combination of factors, including the rising prevalence of sports-related injuries, the growing popularity of high-intensity workouts, and the increasing adoption of recovery techniques among professional athletes and fitness enthusiasts alike.
Hirudoid's products, particularly those focused on topical recovery solutions, have gained traction in both the professional sports and consumer markets. The company's innovative formulations, which leverage advanced ingredients and delivery systems, have resonated with consumers seeking effective recovery options. This has contributed to Hirudoid's market share expansion and brand recognition within the recovery products sector.
The recovery products market can be segmented into various categories, including topical treatments, compression garments, nutrition supplements, and recovery equipment. Hirudoid's primary focus on topical solutions has allowed the company to capitalize on the growing demand for non-invasive, easy-to-use recovery products. This segment of the market is expected to show robust growth, driven by consumer preference for convenient and targeted recovery options.
Geographically, North America and Europe currently dominate the recovery products market, accounting for a significant portion of global sales. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential, presenting opportunities for companies like Hirudoid to expand their global footprint. The increasing disposable income and growing health consciousness in these regions are key factors driving market expansion.
Consumer trends indicate a shift towards more holistic recovery approaches, combining multiple product types and techniques. This trend presents both opportunities and challenges for Hirudoid, as it may need to diversify its product portfolio or form strategic partnerships to offer comprehensive recovery solutions.
The competitive landscape of the recovery products market is characterized by a mix of established pharmaceutical companies, sports equipment manufacturers, and specialized recovery brands. Hirudoid's focus on scientific advancements and product efficacy has helped differentiate its offerings in a crowded marketplace. However, the company faces competition from both large multinational corporations with significant R&D budgets and agile startups leveraging new technologies.
In conclusion, the market analysis for recovery products reveals a promising landscape for Hirudoid's continued growth and innovation. The company's advancements in recovery science position it well to capitalize on the expanding market opportunities, particularly in the topical solutions segment. To maintain its competitive edge, Hirudoid should continue to invest in research and development, explore new market segments, and adapt to evolving consumer preferences in the dynamic recovery products industry.
Current Challenges in Recovery Science
Recovery science has made significant strides in recent years, yet several challenges persist in advancing the field further. One of the primary obstacles is the complexity of biological systems involved in recovery processes. The human body's response to injury or stress is multifaceted, involving intricate interactions between various physiological systems. This complexity makes it difficult to develop comprehensive recovery strategies that address all aspects of healing and regeneration.
Another significant challenge is the variability in individual responses to recovery interventions. Factors such as genetics, age, overall health status, and environmental conditions can greatly influence how a person responds to a particular recovery method. This heterogeneity complicates the development of standardized recovery protocols and necessitates more personalized approaches.
The lack of long-term studies on the efficacy of various recovery techniques poses another hurdle. While many recovery methods show promising short-term results, their long-term effects and potential side effects remain largely unknown. This gap in knowledge makes it challenging for practitioners to make informed decisions about the most appropriate recovery strategies for their patients or clients.
Additionally, the field of recovery science faces challenges in translating laboratory findings into practical, real-world applications. Many promising recovery techniques that show efficacy in controlled laboratory settings may not yield the same results when applied in diverse, real-life scenarios. This translation gap hinders the widespread adoption of innovative recovery methods.
The integration of technology in recovery science, while offering numerous opportunities, also presents challenges. Wearable devices and digital health technologies have the potential to revolutionize recovery monitoring and intervention. However, issues related to data accuracy, privacy concerns, and the interpretation of vast amounts of collected data remain significant obstacles to their effective implementation.
Furthermore, there is a growing need for interdisciplinary collaboration in recovery science. The field spans multiple disciplines, including physiology, biomechanics, nutrition, and psychology. Bridging these diverse areas of expertise to create holistic recovery strategies is a complex task that requires overcoming traditional disciplinary boundaries.
Lastly, the challenge of quantifying recovery progress in a standardized and objective manner persists. While various biomarkers and performance metrics exist, there is still a lack of consensus on the most reliable and comprehensive measures of recovery. This makes it difficult to compare the effectiveness of different recovery methods and to track individual progress accurately.
Another significant challenge is the variability in individual responses to recovery interventions. Factors such as genetics, age, overall health status, and environmental conditions can greatly influence how a person responds to a particular recovery method. This heterogeneity complicates the development of standardized recovery protocols and necessitates more personalized approaches.
The lack of long-term studies on the efficacy of various recovery techniques poses another hurdle. While many recovery methods show promising short-term results, their long-term effects and potential side effects remain largely unknown. This gap in knowledge makes it challenging for practitioners to make informed decisions about the most appropriate recovery strategies for their patients or clients.
Additionally, the field of recovery science faces challenges in translating laboratory findings into practical, real-world applications. Many promising recovery techniques that show efficacy in controlled laboratory settings may not yield the same results when applied in diverse, real-life scenarios. This translation gap hinders the widespread adoption of innovative recovery methods.
The integration of technology in recovery science, while offering numerous opportunities, also presents challenges. Wearable devices and digital health technologies have the potential to revolutionize recovery monitoring and intervention. However, issues related to data accuracy, privacy concerns, and the interpretation of vast amounts of collected data remain significant obstacles to their effective implementation.
Furthermore, there is a growing need for interdisciplinary collaboration in recovery science. The field spans multiple disciplines, including physiology, biomechanics, nutrition, and psychology. Bridging these diverse areas of expertise to create holistic recovery strategies is a complex task that requires overcoming traditional disciplinary boundaries.
Lastly, the challenge of quantifying recovery progress in a standardized and objective manner persists. While various biomarkers and performance metrics exist, there is still a lack of consensus on the most reliable and comprehensive measures of recovery. This makes it difficult to compare the effectiveness of different recovery methods and to track individual progress accurately.
Existing Hirudoid Applications
01 Data recovery and backup systems
Systems and methods for data recovery and backup in computing environments. These solutions focus on protecting data integrity, ensuring business continuity, and minimizing downtime in case of system failures or data loss incidents. They often involve creating redundant copies of data, implementing recovery point objectives, and utilizing various storage technologies.- Data recovery and backup systems: Systems and methods for data recovery and backup in computing environments. These solutions focus on maintaining data integrity, implementing redundancy, and ensuring quick recovery in case of system failures or data loss incidents.
- Network communication and error recovery: Techniques for improving network communication reliability and implementing error recovery mechanisms. These innovations address issues in data transmission, packet loss, and connection stability across various network protocols and architectures.
- Machine learning and AI in system recovery: Application of machine learning and artificial intelligence techniques to enhance system recovery processes. These approaches involve predictive maintenance, anomaly detection, and intelligent decision-making in recovery scenarios.
- Database recovery and management: Methods for efficient database recovery and management, focusing on maintaining data consistency, implementing transaction logging, and ensuring rapid restoration of database systems after failures or crashes.
- Hardware-level recovery mechanisms: Hardware-based solutions for system recovery, including innovations in storage devices, memory systems, and processor architectures. These technologies aim to improve reliability and recovery speed at the hardware level.
02 Network communication and error recovery
Techniques for improving network communication reliability and implementing error recovery mechanisms. These approaches aim to maintain seamless data transmission, handle network disruptions, and ensure quick recovery from communication failures. They may include protocols for packet retransmission, error detection, and correction in various network environments.Expand Specific Solutions03 Machine learning in system recovery
Application of machine learning and artificial intelligence techniques to enhance system recovery processes. These methods involve using predictive algorithms, pattern recognition, and adaptive learning to improve fault detection, automate recovery procedures, and optimize system performance during and after recovery events.Expand Specific Solutions04 Database recovery and management
Specialized techniques for database recovery and management, focusing on maintaining data consistency, implementing transaction logging, and ensuring rapid restoration of database systems. These methods address challenges in handling large-scale data, managing concurrent operations, and minimizing data loss during recovery processes.Expand Specific Solutions05 Hardware-level recovery mechanisms
Hardware-based approaches to system recovery, including techniques implemented at the disk, memory, and processor levels. These solutions focus on rapid error detection, fault isolation, and recovery at the hardware level to minimize system downtime and data loss. They may involve specialized hardware components or firmware-level implementations.Expand Specific Solutions
Key Players in Recovery Industry
The competitive landscape for Hirudoid's advancements in recovery science is evolving rapidly. The industry is in a growth phase, with increasing market size driven by rising demand for innovative recovery solutions. The technology's maturity varies among key players, with companies like Sterix Ltd., AEterna Zentaris GmbH, and Merck Patent GmbH leading in research and development. Universities such as Harvard, MIT, and the University of Southern California are contributing significantly to the field's advancement. Pharmaceutical giants like Bristol Myers Squibb Co. and Janssen Pharmaceutica NV are also investing heavily, indicating the technology's potential. The market is characterized by a mix of established players and emerging startups, suggesting a dynamic and competitive environment with ample opportunities for breakthrough innovations.
Celgene Corp.
Technical Solution: Celgene Corp. has developed a proprietary technology platform for the synthesis and modification of hirudin-based compounds. Their approach involves genetic engineering of recombinant hirudin variants with enhanced stability and bioavailability. These modified hirudins are designed to have improved pharmacokinetic properties, allowing for more effective and longer-lasting anticoagulant effects. Celgene's research also focuses on developing novel delivery systems for these compounds, including sustained-release formulations and targeted delivery to specific tissues.
Strengths: Advanced genetic engineering capabilities, potential for longer-acting anticoagulants. Weaknesses: Potential immunogenicity of modified proteins, high development costs.
Allergan, Inc.
Technical Solution: Allergan, Inc. has been focusing on the development of topical formulations incorporating hirudin for enhanced wound healing and recovery. Their proprietary technology involves the creation of nanoparticle-based delivery systems that can effectively penetrate the skin barrier and deliver hirudin to the target tissues. These formulations are designed to provide localized anticoagulant effects, reduce inflammation, and promote tissue regeneration. Allergan is also exploring the potential of combining hirudin with other bioactive compounds in these topical formulations to create multi-functional recovery products.
Strengths: Expertise in topical drug delivery, potential for non-invasive applications. Weaknesses: Limited systemic effects, potential for variable efficacy depending on skin condition.
Core Innovations in Hirudoid
Modulating nudix homology domain (NHD) with nicotinamide mononucleotide analogs and derivatives of same
PatentWO2018129039A1
Innovation
- Administering nicotinamide mononucleotide or its analogs to modulate the activity of biologically active polypeptides containing Nudix homology domains, thereby influencing protein-protein interactions and cellular processes such as DNA repair, cell survival, and aging.
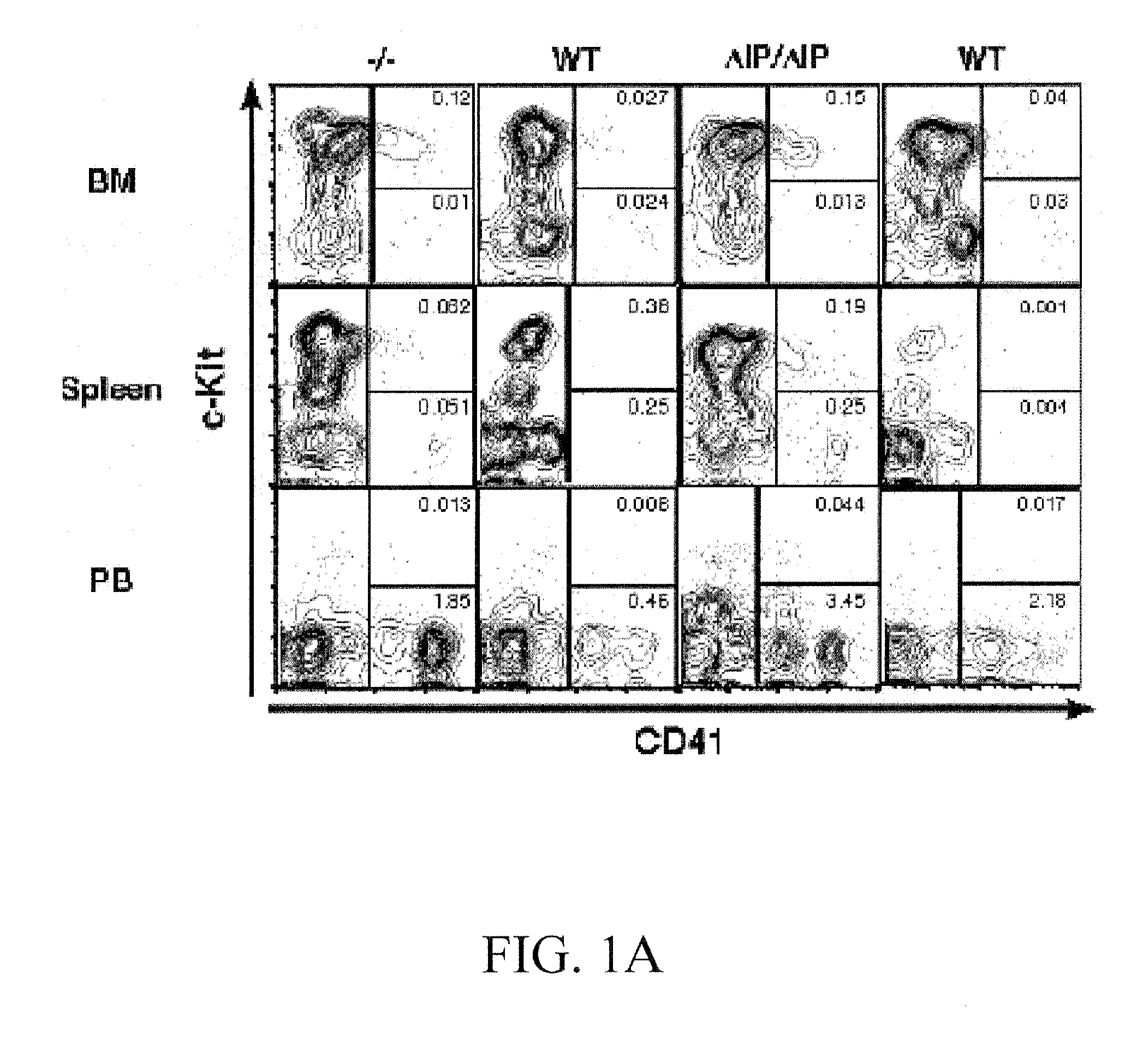
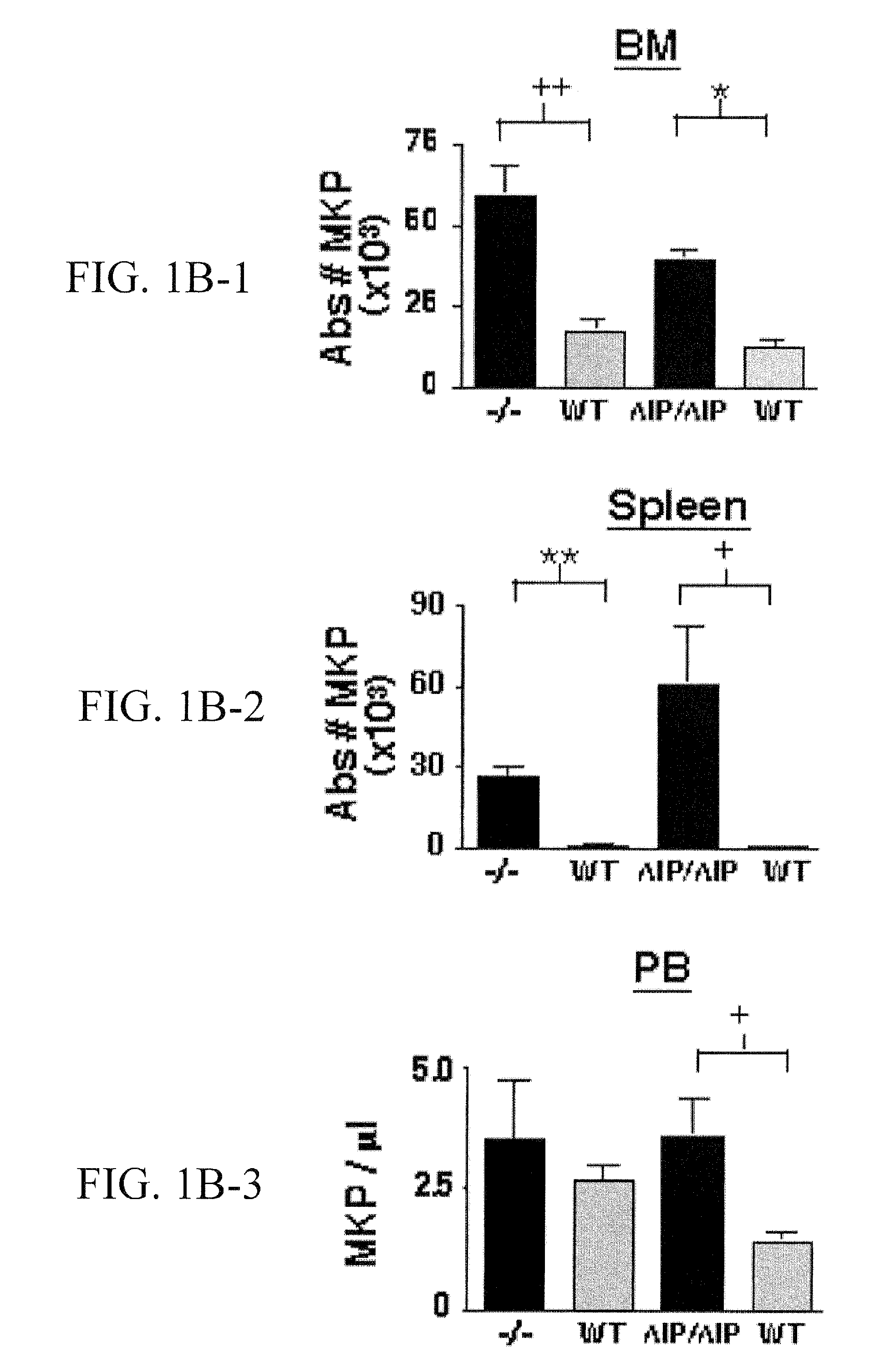
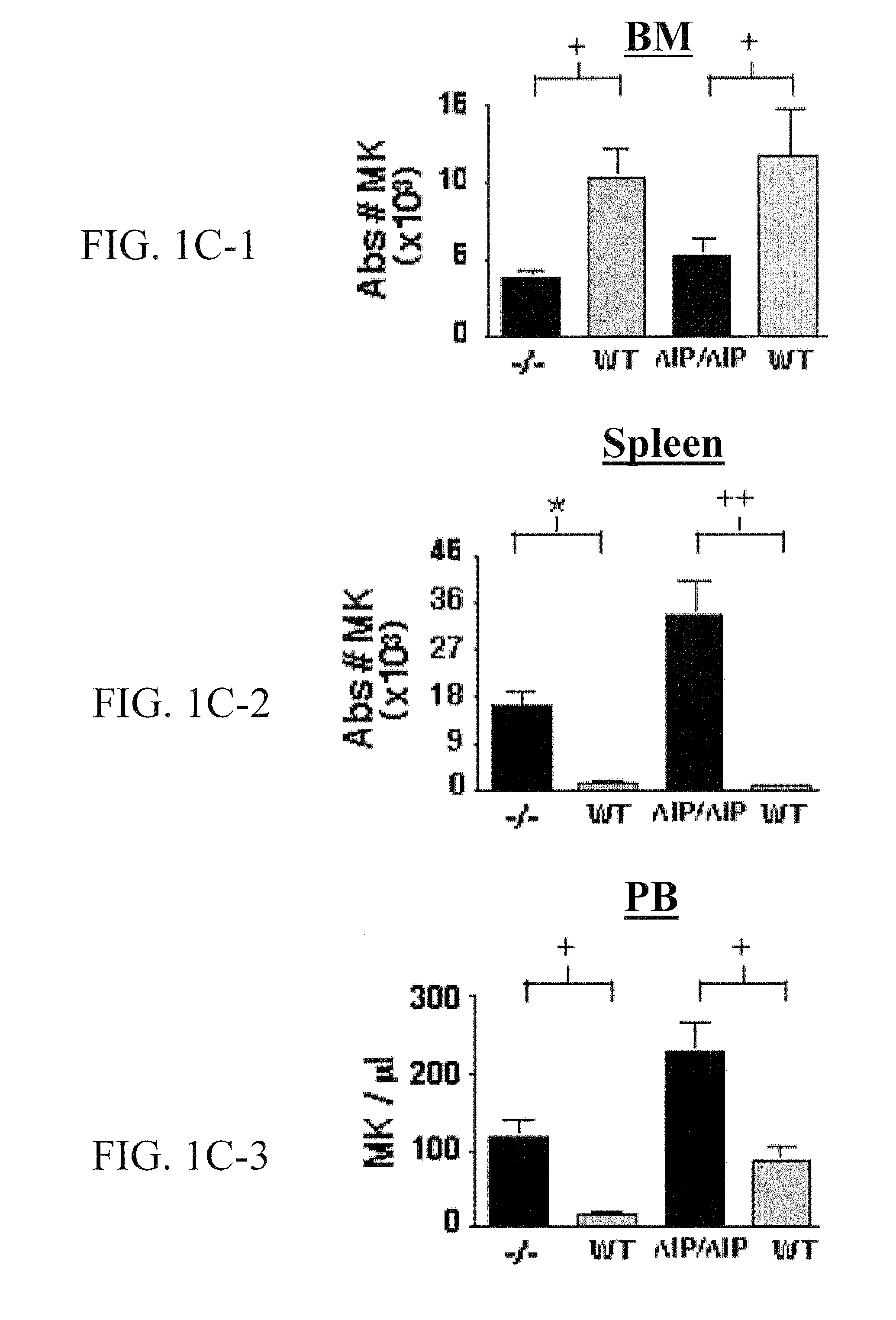
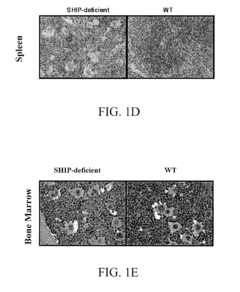
SHIP-Deficiency to Increase Megakaryocyte Progenitor Production
PatentInactiveUS20100260730A1
Innovation
- Inhibiting SHIP gene function using substances like interfering RNA, antisense oligonucleotides, or ribozymes to enhance megakaryocyte and megakaryocyte progenitor production, thereby increasing platelet production.
Clinical Trial Landscape
The clinical trial landscape for Hirudoid's advancements in recovery science has been evolving rapidly in recent years. A comprehensive analysis of the current state reveals several key trends and developments. Firstly, there has been a significant increase in the number of clinical trials focusing on Hirudoid's applications in wound healing and tissue repair. These trials span various phases, from early-stage safety studies to late-stage efficacy trials, indicating a growing interest in the potential therapeutic benefits of Hirudoid.
One notable aspect of the clinical trial landscape is the diversification of research areas. While traditional studies have primarily focused on topical applications for superficial wounds, recent trials have expanded to explore Hirudoid's potential in deeper tissue injuries, post-surgical recovery, and even chronic inflammatory conditions. This broadening scope reflects the scientific community's recognition of Hirudoid's versatile healing properties.
Geographically, the distribution of clinical trials has shown interesting patterns. While European countries, particularly Germany and Switzerland, have historically led in Hirudoid research, there has been a recent surge in trials conducted in Asia, especially in Japan and South Korea. This geographical shift suggests an expanding global interest in Hirudoid's therapeutic potential and may lead to new insights from diverse patient populations.
The design of clinical trials has also evolved, with an increasing emphasis on randomized, double-blind, placebo-controlled studies. This trend towards more rigorous methodologies enhances the reliability and validity of research outcomes, providing stronger evidence for Hirudoid's efficacy. Additionally, there's a growing focus on long-term follow-up studies, aiming to assess the durability of Hirudoid's effects and potential long-term benefits or risks.
Another significant development in the clinical trial landscape is the integration of advanced technologies in research methodologies. The use of high-resolution imaging techniques, molecular biomarkers, and sophisticated data analytics has enabled more precise measurements of Hirudoid's effects on tissue repair and inflammation. These technological advancements are providing deeper insights into the mechanisms of action and are helping to identify optimal dosing regimens and application methods.
Collaboration between academic institutions and pharmaceutical companies has become more prevalent in recent Hirudoid trials. This synergy between scientific expertise and industry resources is accelerating the pace of research and facilitating the translation of laboratory findings into clinical applications. Such collaborations are also instrumental in designing more comprehensive and multi-centered trials, which are crucial for establishing the broad applicability of Hirudoid in diverse clinical settings.
One notable aspect of the clinical trial landscape is the diversification of research areas. While traditional studies have primarily focused on topical applications for superficial wounds, recent trials have expanded to explore Hirudoid's potential in deeper tissue injuries, post-surgical recovery, and even chronic inflammatory conditions. This broadening scope reflects the scientific community's recognition of Hirudoid's versatile healing properties.
Geographically, the distribution of clinical trials has shown interesting patterns. While European countries, particularly Germany and Switzerland, have historically led in Hirudoid research, there has been a recent surge in trials conducted in Asia, especially in Japan and South Korea. This geographical shift suggests an expanding global interest in Hirudoid's therapeutic potential and may lead to new insights from diverse patient populations.
The design of clinical trials has also evolved, with an increasing emphasis on randomized, double-blind, placebo-controlled studies. This trend towards more rigorous methodologies enhances the reliability and validity of research outcomes, providing stronger evidence for Hirudoid's efficacy. Additionally, there's a growing focus on long-term follow-up studies, aiming to assess the durability of Hirudoid's effects and potential long-term benefits or risks.
Another significant development in the clinical trial landscape is the integration of advanced technologies in research methodologies. The use of high-resolution imaging techniques, molecular biomarkers, and sophisticated data analytics has enabled more precise measurements of Hirudoid's effects on tissue repair and inflammation. These technological advancements are providing deeper insights into the mechanisms of action and are helping to identify optimal dosing regimens and application methods.
Collaboration between academic institutions and pharmaceutical companies has become more prevalent in recent Hirudoid trials. This synergy between scientific expertise and industry resources is accelerating the pace of research and facilitating the translation of laboratory findings into clinical applications. Such collaborations are also instrumental in designing more comprehensive and multi-centered trials, which are crucial for establishing the broad applicability of Hirudoid in diverse clinical settings.
Regulatory Considerations
The regulatory landscape surrounding Hirudoid's advancements in recovery science is complex and multifaceted, requiring careful navigation to ensure compliance and market access. As a medical product, Hirudoid falls under the purview of various regulatory bodies, primarily the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in the European Union.
In the United States, Hirudoid is classified as a topical heparinoid, which places it under the FDA's drug regulatory framework. This classification necessitates rigorous clinical trials to demonstrate safety and efficacy before market approval can be granted. The FDA's approval process typically involves multiple phases of clinical trials, with each phase designed to assess different aspects of the product's performance and safety profile.
European regulations, governed by the EMA, follow a similar pattern but may have additional requirements specific to the EU market. The EMA's centralized procedure for drug approval allows for a single marketing authorization applicable across all EU member states, streamlining the process for widespread distribution.
Both regulatory bodies emphasize the importance of Good Manufacturing Practices (GMP) in the production of Hirudoid. Adherence to GMP standards ensures consistent quality and safety of the product throughout its lifecycle. Regular inspections of manufacturing facilities are conducted to verify compliance with these standards.
Post-market surveillance is another critical aspect of regulatory compliance for Hirudoid. Manufacturers are required to monitor and report any adverse events or safety concerns that arise after the product has been released to the market. This ongoing vigilance helps to ensure the long-term safety and efficacy of the product.
As Hirudoid advances in recovery science, any significant changes to its formulation or intended use may require additional regulatory review and approval. This could involve submitting new clinical data or conducting additional studies to support the proposed changes.
Regulatory considerations also extend to labeling and marketing claims. All product information, including packaging, inserts, and promotional materials, must accurately reflect the approved indications and safety information. Regulatory bodies closely scrutinize these materials to prevent misleading or exaggerated claims.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to streamline regulatory processes across different regions. These initiatives can potentially expedite global market access for products like Hirudoid by reducing duplicative testing and documentation requirements.
In the United States, Hirudoid is classified as a topical heparinoid, which places it under the FDA's drug regulatory framework. This classification necessitates rigorous clinical trials to demonstrate safety and efficacy before market approval can be granted. The FDA's approval process typically involves multiple phases of clinical trials, with each phase designed to assess different aspects of the product's performance and safety profile.
European regulations, governed by the EMA, follow a similar pattern but may have additional requirements specific to the EU market. The EMA's centralized procedure for drug approval allows for a single marketing authorization applicable across all EU member states, streamlining the process for widespread distribution.
Both regulatory bodies emphasize the importance of Good Manufacturing Practices (GMP) in the production of Hirudoid. Adherence to GMP standards ensures consistent quality and safety of the product throughout its lifecycle. Regular inspections of manufacturing facilities are conducted to verify compliance with these standards.
Post-market surveillance is another critical aspect of regulatory compliance for Hirudoid. Manufacturers are required to monitor and report any adverse events or safety concerns that arise after the product has been released to the market. This ongoing vigilance helps to ensure the long-term safety and efficacy of the product.
As Hirudoid advances in recovery science, any significant changes to its formulation or intended use may require additional regulatory review and approval. This could involve submitting new clinical data or conducting additional studies to support the proposed changes.
Regulatory considerations also extend to labeling and marketing claims. All product information, including packaging, inserts, and promotional materials, must accurately reflect the approved indications and safety information. Regulatory bodies closely scrutinize these materials to prevent misleading or exaggerated claims.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to streamline regulatory processes across different regions. These initiatives can potentially expedite global market access for products like Hirudoid by reducing duplicative testing and documentation requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!