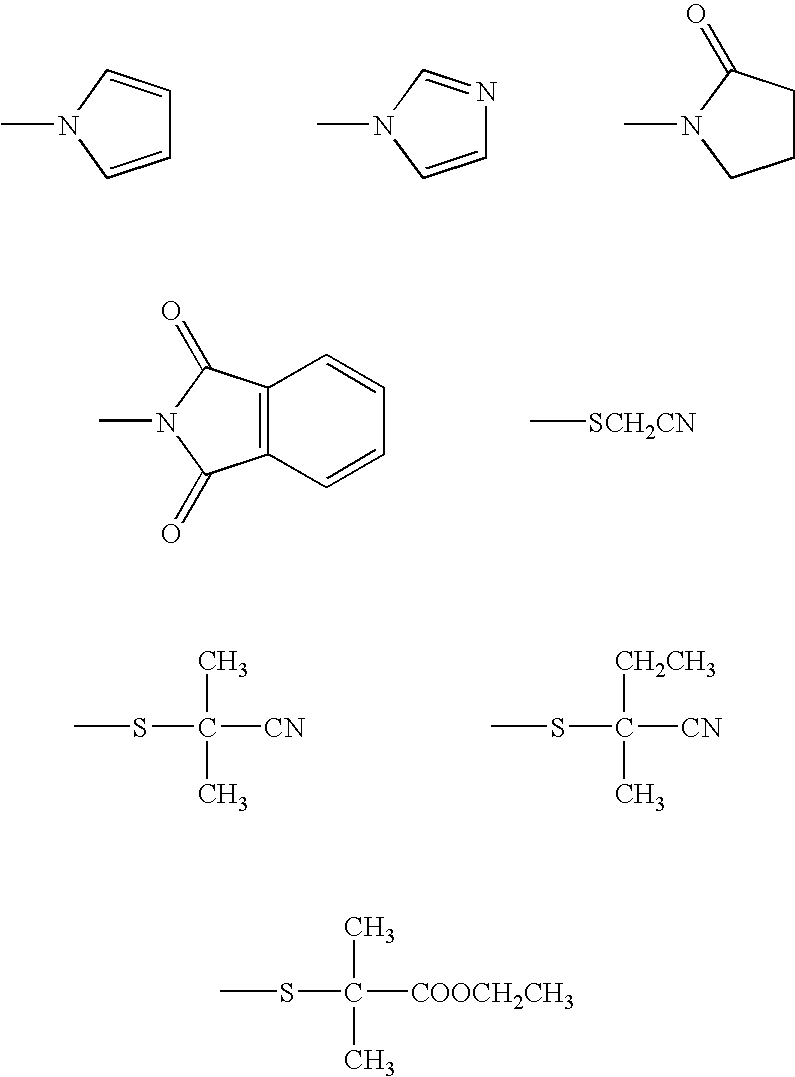
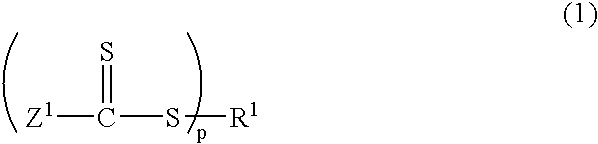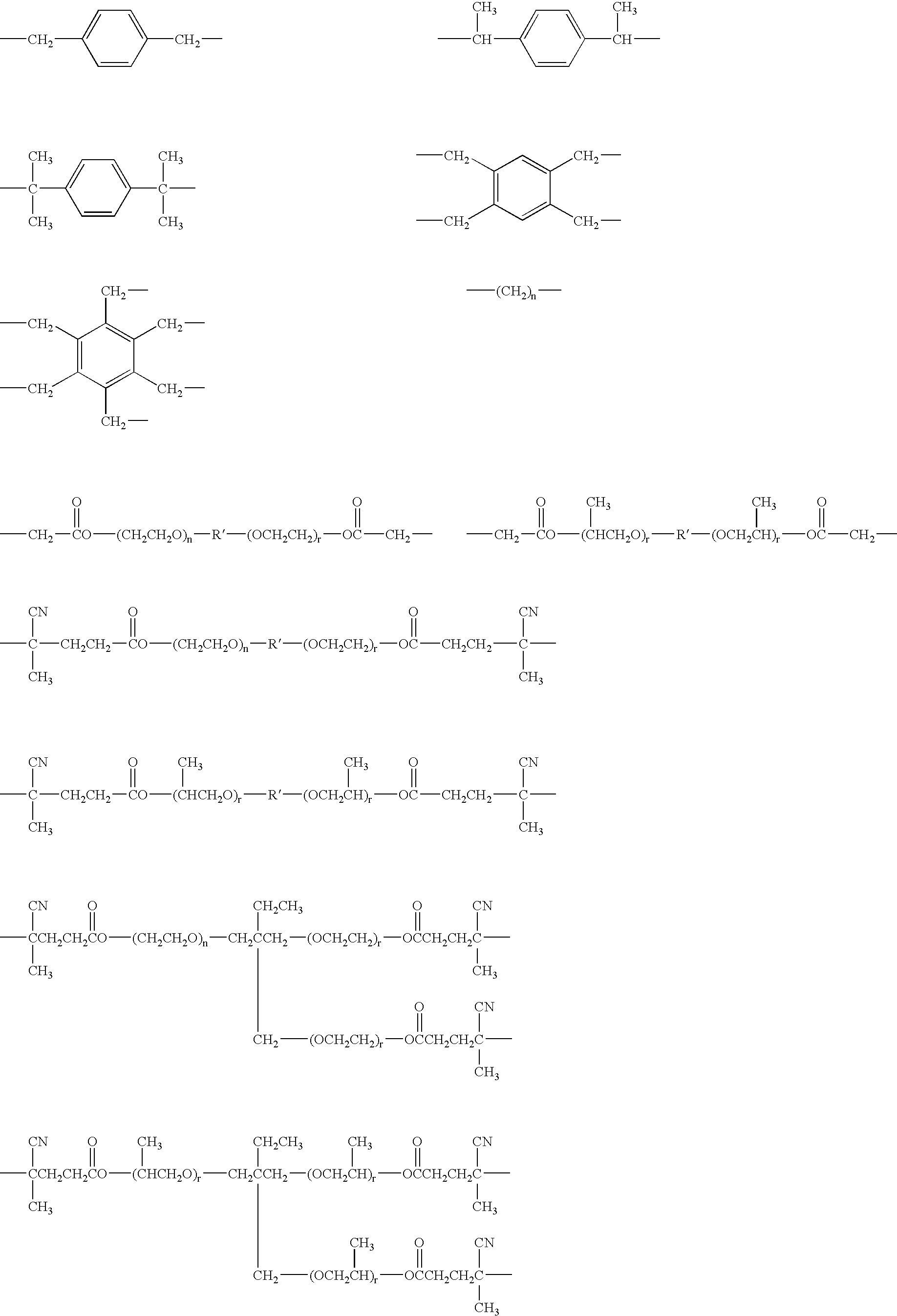How Heptane Affects Monomer Solubility in Solution Polymerization
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Heptane and Monomer Solubility Background
Solution polymerization is a widely used technique in the production of various polymers, where monomers are dissolved in a solvent and then polymerized. The choice of solvent plays a crucial role in determining the solubility of monomers and the overall efficiency of the polymerization process. Heptane, a straight-chain alkane with seven carbon atoms, is frequently employed as a solvent in solution polymerization due to its unique properties and effects on monomer solubility.
The interaction between heptane and monomers is governed by fundamental principles of solubility and intermolecular forces. Heptane, being a non-polar solvent, exhibits limited solubility for polar monomers but can effectively dissolve non-polar or slightly polar monomers. This selectivity in solubility is primarily attributed to the "like dissolves like" principle, where substances with similar polarity tend to be more soluble in each other.
The solubility of monomers in heptane is influenced by several factors, including temperature, pressure, and the presence of other components in the solution. As temperature increases, the solubility of most monomers in heptane generally improves due to increased molecular motion and weakening of intermolecular forces. Pressure effects are typically less significant in liquid-phase systems but can become important in high-pressure polymerization processes.
Heptane's impact on monomer solubility extends beyond simple dissolution. It can affect the conformation and reactivity of monomers in solution, potentially influencing the kinetics and thermodynamics of the polymerization reaction. The solvation of monomers by heptane can alter their electronic distribution and accessibility, which may, in turn, affect their ability to participate in chain growth or termination reactions.
The use of heptane as a solvent in solution polymerization offers several advantages. Its low boiling point (98.4°C) facilitates easy removal and recovery after polymerization, reducing energy costs and environmental impact. Additionally, heptane's non-polar nature makes it an excellent medium for controlling the molecular weight of polymers through chain transfer reactions.
However, the limited solubility of polar monomers in heptane can pose challenges in certain polymerization systems. This limitation has led to the development of various strategies to enhance monomer solubility, such as the use of co-solvents, surfactants, or temperature modulation. Understanding these solubility dynamics is crucial for optimizing reaction conditions and achieving desired polymer properties.
The historical development of solution polymerization techniques has seen a progressive refinement in the understanding of solvent-monomer interactions. Early studies focused primarily on empirical observations, while modern research employs advanced spectroscopic and computational methods to elucidate the molecular-level interactions between heptane and various monomers. This evolving knowledge base continues to drive innovations in polymer synthesis and processing technologies.
The interaction between heptane and monomers is governed by fundamental principles of solubility and intermolecular forces. Heptane, being a non-polar solvent, exhibits limited solubility for polar monomers but can effectively dissolve non-polar or slightly polar monomers. This selectivity in solubility is primarily attributed to the "like dissolves like" principle, where substances with similar polarity tend to be more soluble in each other.
The solubility of monomers in heptane is influenced by several factors, including temperature, pressure, and the presence of other components in the solution. As temperature increases, the solubility of most monomers in heptane generally improves due to increased molecular motion and weakening of intermolecular forces. Pressure effects are typically less significant in liquid-phase systems but can become important in high-pressure polymerization processes.
Heptane's impact on monomer solubility extends beyond simple dissolution. It can affect the conformation and reactivity of monomers in solution, potentially influencing the kinetics and thermodynamics of the polymerization reaction. The solvation of monomers by heptane can alter their electronic distribution and accessibility, which may, in turn, affect their ability to participate in chain growth or termination reactions.
The use of heptane as a solvent in solution polymerization offers several advantages. Its low boiling point (98.4°C) facilitates easy removal and recovery after polymerization, reducing energy costs and environmental impact. Additionally, heptane's non-polar nature makes it an excellent medium for controlling the molecular weight of polymers through chain transfer reactions.
However, the limited solubility of polar monomers in heptane can pose challenges in certain polymerization systems. This limitation has led to the development of various strategies to enhance monomer solubility, such as the use of co-solvents, surfactants, or temperature modulation. Understanding these solubility dynamics is crucial for optimizing reaction conditions and achieving desired polymer properties.
The historical development of solution polymerization techniques has seen a progressive refinement in the understanding of solvent-monomer interactions. Early studies focused primarily on empirical observations, while modern research employs advanced spectroscopic and computational methods to elucidate the molecular-level interactions between heptane and various monomers. This evolving knowledge base continues to drive innovations in polymer synthesis and processing technologies.
Market Analysis for Heptane-Based Polymerization
The market for heptane-based polymerization has shown significant growth in recent years, driven by increasing demand for high-performance polymers across various industries. The global market size for heptane-based polymerization was estimated at $2.3 billion in 2022, with a projected compound annual growth rate (CAGR) of 5.7% from 2023 to 2028.
The automotive sector represents the largest end-user segment for heptane-based polymerization products, accounting for approximately 35% of the market share. This is primarily due to the growing demand for lightweight and durable materials in vehicle manufacturing. The construction industry follows closely, with a market share of around 28%, driven by the need for weather-resistant and long-lasting polymers in building materials.
Geographically, Asia-Pacific dominates the market, holding a 42% share, with China and India being the major contributors. North America and Europe follow with market shares of 28% and 22%, respectively. The Middle East and Africa region is expected to witness the fastest growth in the coming years, with a CAGR of 6.8%, due to increasing industrialization and infrastructure development.
Key market drivers include the rising demand for eco-friendly and sustainable polymers, technological advancements in polymerization processes, and the expanding applications of heptane-based polymers in emerging industries such as 3D printing and renewable energy. However, the market faces challenges such as volatile raw material prices and stringent environmental regulations.
The competitive landscape is characterized by the presence of both global players and regional manufacturers. Major companies in the market include BASF SE, DowDuPont, LyondellBasell Industries, and Exxon Mobil Corporation. These companies are focusing on research and development to improve product quality and expand their product portfolios.
Looking ahead, the market is expected to witness further growth opportunities in the development of bio-based heptane alternatives and the increasing adoption of heptane-based polymers in the healthcare and electronics industries. The shift towards circular economy principles is also likely to drive innovation in recycling and reprocessing technologies for heptane-based polymers.
The automotive sector represents the largest end-user segment for heptane-based polymerization products, accounting for approximately 35% of the market share. This is primarily due to the growing demand for lightweight and durable materials in vehicle manufacturing. The construction industry follows closely, with a market share of around 28%, driven by the need for weather-resistant and long-lasting polymers in building materials.
Geographically, Asia-Pacific dominates the market, holding a 42% share, with China and India being the major contributors. North America and Europe follow with market shares of 28% and 22%, respectively. The Middle East and Africa region is expected to witness the fastest growth in the coming years, with a CAGR of 6.8%, due to increasing industrialization and infrastructure development.
Key market drivers include the rising demand for eco-friendly and sustainable polymers, technological advancements in polymerization processes, and the expanding applications of heptane-based polymers in emerging industries such as 3D printing and renewable energy. However, the market faces challenges such as volatile raw material prices and stringent environmental regulations.
The competitive landscape is characterized by the presence of both global players and regional manufacturers. Major companies in the market include BASF SE, DowDuPont, LyondellBasell Industries, and Exxon Mobil Corporation. These companies are focusing on research and development to improve product quality and expand their product portfolios.
Looking ahead, the market is expected to witness further growth opportunities in the development of bio-based heptane alternatives and the increasing adoption of heptane-based polymers in the healthcare and electronics industries. The shift towards circular economy principles is also likely to drive innovation in recycling and reprocessing technologies for heptane-based polymers.
Current Challenges in Solution Polymerization
Solution polymerization is a widely used technique in the production of various polymers, but it faces several significant challenges in current industrial applications. One of the primary issues is the control of monomer solubility, particularly when using solvents like heptane. The solubility of monomers in the reaction medium directly impacts the polymerization kinetics, molecular weight distribution, and overall polymer properties.
A major challenge lies in maintaining consistent monomer concentration throughout the polymerization process. As the reaction progresses, the solubility of monomers can change, leading to heterogeneous reaction conditions. This variability can result in inconsistent polymer properties and reduced product quality. The use of heptane as a solvent further complicates this issue due to its non-polar nature, which can limit the solubility of certain monomers, especially those with polar functional groups.
Temperature control presents another significant challenge in solution polymerization. The heat generated during the exothermic polymerization reaction can cause localized temperature increases, potentially leading to hot spots within the reactor. These temperature fluctuations can affect monomer solubility and reaction rates, resulting in uneven polymer growth and potential side reactions. The presence of heptane, with its relatively low boiling point, adds complexity to temperature management strategies.
The formation of high molecular weight polymers often leads to increased solution viscosity, which can hinder efficient mixing and heat transfer. This phenomenon is particularly pronounced in heptane-based systems, where polymer-solvent interactions may be less favorable. As viscosity increases, maintaining uniform monomer distribution becomes more challenging, potentially leading to compositional drift in copolymerization reactions.
Another critical challenge is the management of chain transfer reactions, which can significantly impact the molecular weight and distribution of the final polymer. Heptane, being a hydrocarbon solvent, may participate in chain transfer reactions, affecting the control over polymer architecture and properties. Balancing the desired polymer characteristics with the inherent limitations of the solvent system requires careful consideration of reaction conditions and catalyst selection.
The recovery and purification of the final polymer product from the heptane solution pose additional challenges. Efficient solvent removal and polymer isolation are crucial for maintaining product quality and minimizing environmental impact. The development of cost-effective and environmentally friendly separation techniques remains an ongoing area of research in solution polymerization processes.
A major challenge lies in maintaining consistent monomer concentration throughout the polymerization process. As the reaction progresses, the solubility of monomers can change, leading to heterogeneous reaction conditions. This variability can result in inconsistent polymer properties and reduced product quality. The use of heptane as a solvent further complicates this issue due to its non-polar nature, which can limit the solubility of certain monomers, especially those with polar functional groups.
Temperature control presents another significant challenge in solution polymerization. The heat generated during the exothermic polymerization reaction can cause localized temperature increases, potentially leading to hot spots within the reactor. These temperature fluctuations can affect monomer solubility and reaction rates, resulting in uneven polymer growth and potential side reactions. The presence of heptane, with its relatively low boiling point, adds complexity to temperature management strategies.
The formation of high molecular weight polymers often leads to increased solution viscosity, which can hinder efficient mixing and heat transfer. This phenomenon is particularly pronounced in heptane-based systems, where polymer-solvent interactions may be less favorable. As viscosity increases, maintaining uniform monomer distribution becomes more challenging, potentially leading to compositional drift in copolymerization reactions.
Another critical challenge is the management of chain transfer reactions, which can significantly impact the molecular weight and distribution of the final polymer. Heptane, being a hydrocarbon solvent, may participate in chain transfer reactions, affecting the control over polymer architecture and properties. Balancing the desired polymer characteristics with the inherent limitations of the solvent system requires careful consideration of reaction conditions and catalyst selection.
The recovery and purification of the final polymer product from the heptane solution pose additional challenges. Efficient solvent removal and polymer isolation are crucial for maintaining product quality and minimizing environmental impact. The development of cost-effective and environmentally friendly separation techniques remains an ongoing area of research in solution polymerization processes.
Existing Methods for Solubility Enhancement
01 Solubility of heptane monomers in various solvents
The solubility of heptane monomers varies in different solvents, which is crucial for their use in various applications. Understanding the solubility characteristics helps in selecting appropriate solvents for processes involving heptane monomers, such as polymerization reactions or purification methods.- Solubility of heptane monomers in various solvents: The solubility of heptane monomers varies in different solvents, which is crucial for their use in various applications. Understanding the solubility characteristics helps in selecting appropriate solvents for processes involving heptane monomers, such as polymerization reactions or purification methods.
- Effect of temperature on heptane monomer solubility: Temperature plays a significant role in the solubility of heptane monomers. As temperature increases, the solubility of heptane monomers generally increases in most solvents. This relationship is important for optimizing reaction conditions and separation processes involving heptane monomers.
- Solubility of heptane monomers in polymer matrices: The solubility of heptane monomers in polymer matrices is relevant for applications such as polymer blending and composite materials. Understanding this solubility helps in predicting the behavior of heptane monomers during processing and in the final product properties.
- Influence of additives on heptane monomer solubility: Various additives can affect the solubility of heptane monomers in different media. These additives may include surfactants, co-solvents, or specific chemical agents that can enhance or reduce the solubility of heptane monomers, which is important for formulation development and process optimization.
- Solubility-related applications of heptane monomers: The solubility characteristics of heptane monomers are utilized in various applications, including their use as solvents, in polymerization reactions, and in the production of specialty chemicals. Understanding and controlling the solubility of heptane monomers is crucial for these applications and can lead to improved product quality and process efficiency.
02 Effect of temperature on heptane monomer solubility
Temperature plays a significant role in the solubility of heptane monomers. As temperature increases, the solubility of heptane monomers generally increases in most solvents. This relationship is important for optimizing reaction conditions and separation processes involving heptane monomers.Expand Specific Solutions03 Solubility enhancement techniques for heptane monomers
Various techniques can be employed to enhance the solubility of heptane monomers in different media. These may include the use of co-solvents, surfactants, or other additives that can improve the miscibility of heptane monomers with other substances, facilitating their use in diverse applications.Expand Specific Solutions04 Solubility-related applications of heptane monomers
The solubility properties of heptane monomers are utilized in various applications, such as in the production of polymers, adhesives, and coatings. Understanding and controlling the solubility of heptane monomers is crucial for achieving desired product characteristics and optimizing manufacturing processes.Expand Specific Solutions05 Environmental and safety considerations related to heptane monomer solubility
The solubility of heptane monomers in different media has implications for environmental and safety concerns. This includes their potential for environmental persistence, bioaccumulation, and the development of safe handling and disposal methods. Understanding these aspects is crucial for regulatory compliance and sustainable use of heptane monomers.Expand Specific Solutions
Key Players in Polymer Industry
The competitive landscape for "How Heptane Affects Monomer Solubility in Solution Polymerization" is in a mature stage, with established players like JSR Corp., Kaneka Corp., and Sekisui Chemical Co., Ltd. leading research and development efforts. The market size is significant, driven by the polymer industry's demand for efficient polymerization processes. Technologically, the field is advanced, with companies like China Petroleum & Chemical Corp. and ExxonMobil Chemical Patents, Inc. continuously innovating. Academic institutions such as Beijing University of Chemical Technology and East China Normal University contribute to fundamental research, enhancing the overall technological maturity of this area.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to enhance monomer solubility in solution polymerization using heptane as a solvent. Their method involves a two-stage process: first, they use a controlled amount of heptane to create a homogeneous solution with the monomer, then gradually increase the heptane concentration to induce phase separation and control polymer molecular weight[1]. This technique allows for better control of reaction kinetics and polymer properties. Sinopec has also implemented in-situ FTIR spectroscopy to monitor the solubility changes in real-time, enabling precise adjustments to the heptane-monomer ratio throughout the polymerization process[3].
Strengths: Improved control over polymer properties, enhanced reaction efficiency. Weaknesses: May require specialized equipment for precise solvent control and monitoring.
Equistar Chemicals LP
Technical Solution: Equistar Chemicals has developed an innovative approach to utilizing heptane in solution polymerization, focusing on its impact on monomer solubility. Their method involves a gradient heptane addition technique, where the concentration of heptane is carefully controlled throughout the polymerization process. This allows for fine-tuning of the solubility parameters, resulting in improved control over polymer molecular weight and distribution[5]. Equistar has also implemented advanced computational fluid dynamics models to predict and optimize the heptane-monomer interactions in their reactors, leading to enhanced process efficiency and product quality[6].
Strengths: Precise control over polymer properties, potential for energy savings in the polymerization process. Weaknesses: May require sophisticated process control systems and extensive operator training.
Core Research on Heptane-Monomer Interactions
Solution polymerization process
PatentInactiveUS4271060A
Innovation
- Incorporating a volatile inert hydrocarbon diluent with 3 to 5 carbon atoms into the polymerization process allows for increased flashing of hydrocarbon vapor, reducing the amount of solvent to be removed by steam stripping and enabling solvent recycling without drying, thereby reducing energy consumption and facilitating polymer concentration.
Polyurethane polymer
PatentInactiveUS6992138B2
Innovation
- A polyurethane polymer is produced using a mercapto group-containing vinyl polymer, prepared by reversible addition-fragmentation chain transfer (RAFT) polymerization, combined with an organic polyisocyanate, to enhance the polymer's resistance properties and simplify production.
Environmental Impact of Heptane Use
The use of heptane in solution polymerization processes has significant environmental implications that warrant careful consideration. As a volatile organic compound (VOC), heptane can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. This poses potential risks to both human health and ecosystems, particularly in urban areas where industrial activities are concentrated.
Heptane's low water solubility and high mobility in soil make it a potential contaminant of groundwater resources if not properly managed. Accidental spills or improper disposal can lead to soil and water pollution, affecting aquatic life and potentially entering the food chain. The bioaccumulation potential of heptane in aquatic organisms further amplifies its environmental impact.
From a lifecycle perspective, the production and transportation of heptane also contribute to its overall environmental footprint. The petrochemical processes involved in heptane manufacture consume significant energy and resources, leading to greenhouse gas emissions and other environmental burdens. Additionally, the transportation of heptane to polymerization facilities adds to carbon emissions and increases the risk of accidental releases during transit.
In the context of solution polymerization, the recovery and recycling of heptane present both challenges and opportunities. Efficient solvent recovery systems can significantly reduce emissions and waste, but these processes themselves require energy and may generate secondary pollutants. The trade-off between environmental impact and process efficiency must be carefully balanced.
Regulatory frameworks worldwide are increasingly focusing on the environmental impact of industrial solvents like heptane. Stringent emission controls, waste management protocols, and reporting requirements are being implemented to mitigate the environmental risks associated with heptane use. This regulatory landscape is driving innovation in greener alternatives and more sustainable polymerization processes.
The search for environmentally friendly alternatives to heptane in solution polymerization is an active area of research. Bio-based solvents, supercritical CO2, and water-based systems are being explored as potential substitutes. However, these alternatives often face challenges in matching the performance and cost-effectiveness of heptane, highlighting the complexity of balancing environmental concerns with technical and economic feasibility.
As industries strive for sustainability, the environmental impact of heptane use in solution polymerization remains a critical consideration. Ongoing efforts to optimize processes, improve recovery techniques, and develop greener alternatives are essential for reducing the environmental footprint of polymer production while maintaining product quality and economic viability.
Heptane's low water solubility and high mobility in soil make it a potential contaminant of groundwater resources if not properly managed. Accidental spills or improper disposal can lead to soil and water pollution, affecting aquatic life and potentially entering the food chain. The bioaccumulation potential of heptane in aquatic organisms further amplifies its environmental impact.
From a lifecycle perspective, the production and transportation of heptane also contribute to its overall environmental footprint. The petrochemical processes involved in heptane manufacture consume significant energy and resources, leading to greenhouse gas emissions and other environmental burdens. Additionally, the transportation of heptane to polymerization facilities adds to carbon emissions and increases the risk of accidental releases during transit.
In the context of solution polymerization, the recovery and recycling of heptane present both challenges and opportunities. Efficient solvent recovery systems can significantly reduce emissions and waste, but these processes themselves require energy and may generate secondary pollutants. The trade-off between environmental impact and process efficiency must be carefully balanced.
Regulatory frameworks worldwide are increasingly focusing on the environmental impact of industrial solvents like heptane. Stringent emission controls, waste management protocols, and reporting requirements are being implemented to mitigate the environmental risks associated with heptane use. This regulatory landscape is driving innovation in greener alternatives and more sustainable polymerization processes.
The search for environmentally friendly alternatives to heptane in solution polymerization is an active area of research. Bio-based solvents, supercritical CO2, and water-based systems are being explored as potential substitutes. However, these alternatives often face challenges in matching the performance and cost-effectiveness of heptane, highlighting the complexity of balancing environmental concerns with technical and economic feasibility.
As industries strive for sustainability, the environmental impact of heptane use in solution polymerization remains a critical consideration. Ongoing efforts to optimize processes, improve recovery techniques, and develop greener alternatives are essential for reducing the environmental footprint of polymer production while maintaining product quality and economic viability.
Scalability and Industrial Applications
The scalability and industrial applications of heptane's effects on monomer solubility in solution polymerization are of significant importance in the polymer manufacturing sector. The use of heptane as a solvent in solution polymerization processes has shown promising results in terms of enhancing monomer solubility, which directly impacts the efficiency and quality of polymer production.
In large-scale industrial settings, the ability to control monomer solubility using heptane offers several advantages. Firstly, it allows for better control over the polymerization reaction, resulting in more consistent polymer properties and improved product quality. This is particularly crucial in industries such as automotive, packaging, and consumer goods, where polymer performance and uniformity are critical.
The scalability of heptane-based solution polymerization processes has been demonstrated in various industrial applications. For instance, in the production of synthetic rubbers, heptane has been successfully employed to enhance the solubility of monomers like butadiene and styrene. This has led to increased production rates and improved product characteristics, such as better elasticity and durability.
Furthermore, the use of heptane in solution polymerization has shown potential for reducing energy consumption in large-scale operations. By improving monomer solubility, the overall reaction efficiency is enhanced, potentially leading to shorter reaction times and lower energy requirements. This aspect is particularly attractive for industries seeking to optimize their production processes and reduce operational costs.
In the field of specialty polymers, the scalability of heptane-based processes has opened up new possibilities for producing high-performance materials. For example, in the manufacture of advanced coatings and adhesives, the improved monomer solubility facilitated by heptane has enabled the production of polymers with enhanced properties, such as increased adhesion strength and chemical resistance.
However, it is important to note that scaling up heptane-based solution polymerization processes also presents challenges. These include the need for specialized equipment to handle large volumes of heptane safely, as well as considerations for solvent recovery and recycling to ensure economic viability and environmental sustainability.
As industries continue to seek more efficient and sustainable production methods, the role of heptane in enhancing monomer solubility is likely to gain further attention. Research into optimizing heptane-based processes for different polymer types and exploring hybrid solvent systems could lead to even broader industrial applications and improved scalability in the future.
In large-scale industrial settings, the ability to control monomer solubility using heptane offers several advantages. Firstly, it allows for better control over the polymerization reaction, resulting in more consistent polymer properties and improved product quality. This is particularly crucial in industries such as automotive, packaging, and consumer goods, where polymer performance and uniformity are critical.
The scalability of heptane-based solution polymerization processes has been demonstrated in various industrial applications. For instance, in the production of synthetic rubbers, heptane has been successfully employed to enhance the solubility of monomers like butadiene and styrene. This has led to increased production rates and improved product characteristics, such as better elasticity and durability.
Furthermore, the use of heptane in solution polymerization has shown potential for reducing energy consumption in large-scale operations. By improving monomer solubility, the overall reaction efficiency is enhanced, potentially leading to shorter reaction times and lower energy requirements. This aspect is particularly attractive for industries seeking to optimize their production processes and reduce operational costs.
In the field of specialty polymers, the scalability of heptane-based processes has opened up new possibilities for producing high-performance materials. For example, in the manufacture of advanced coatings and adhesives, the improved monomer solubility facilitated by heptane has enabled the production of polymers with enhanced properties, such as increased adhesion strength and chemical resistance.
However, it is important to note that scaling up heptane-based solution polymerization processes also presents challenges. These include the need for specialized equipment to handle large volumes of heptane safely, as well as considerations for solvent recovery and recycling to ensure economic viability and environmental sustainability.
As industries continue to seek more efficient and sustainable production methods, the role of heptane in enhancing monomer solubility is likely to gain further attention. Research into optimizing heptane-based processes for different polymer types and exploring hybrid solvent systems could lead to even broader industrial applications and improved scalability in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!