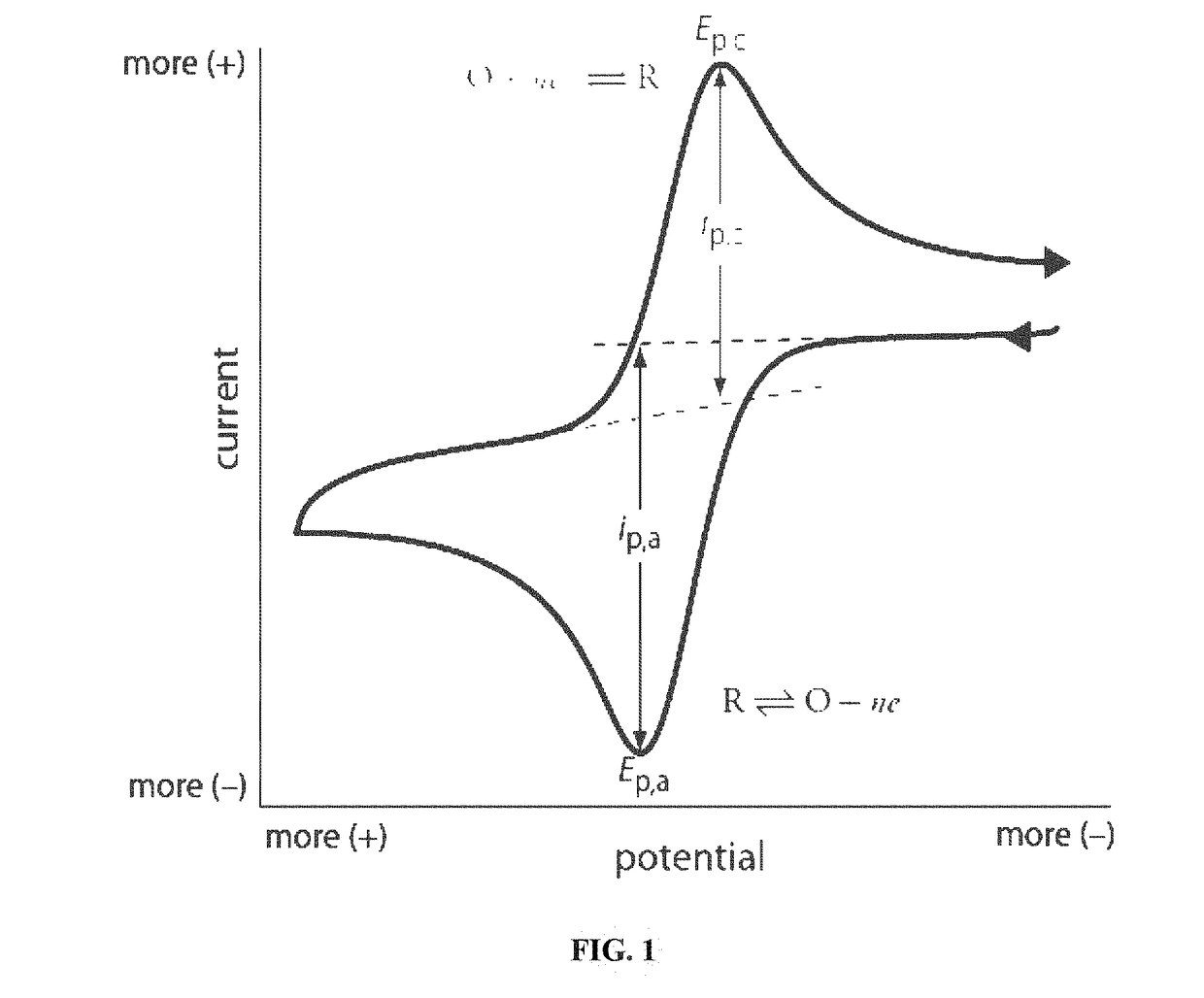
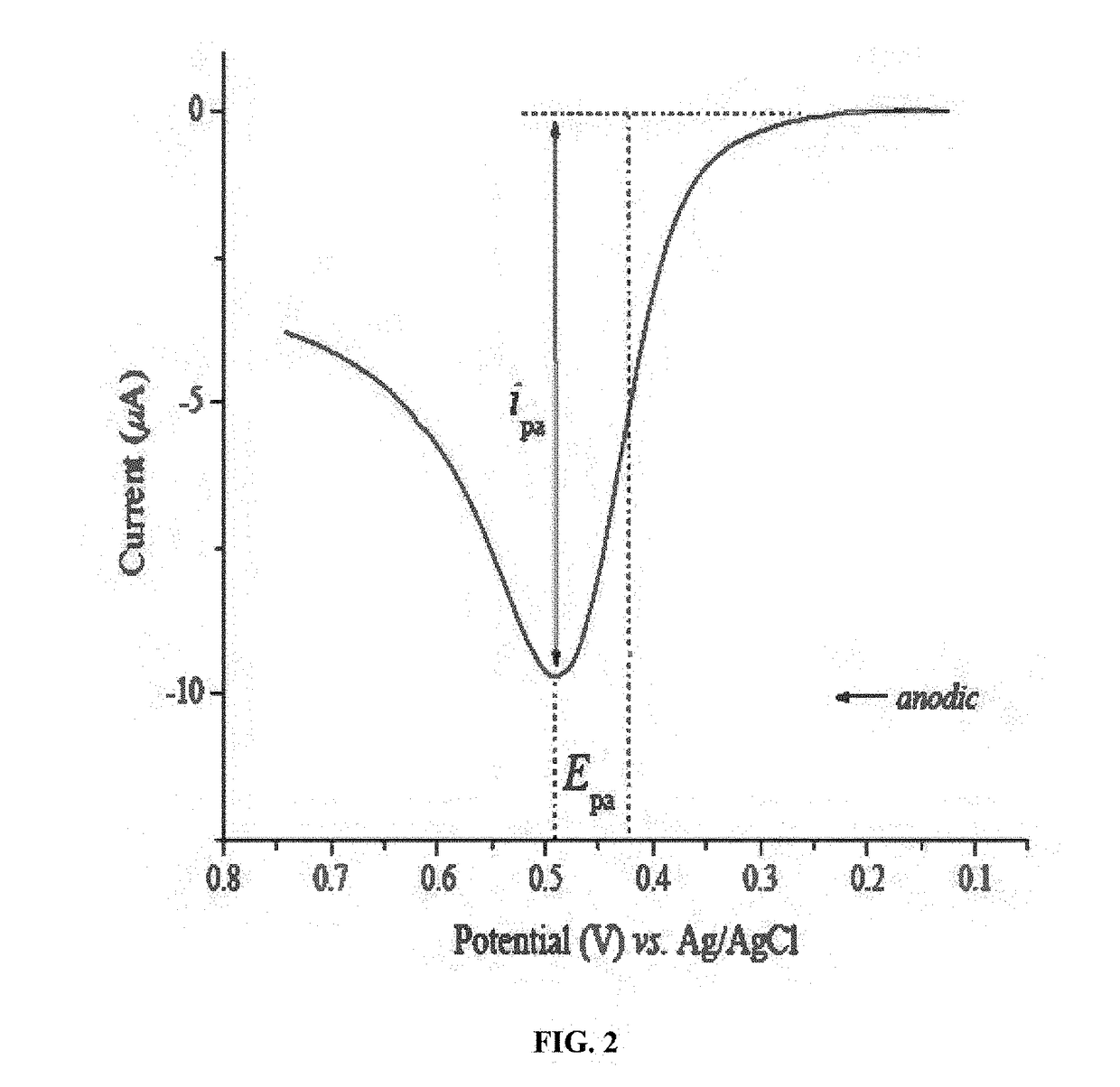
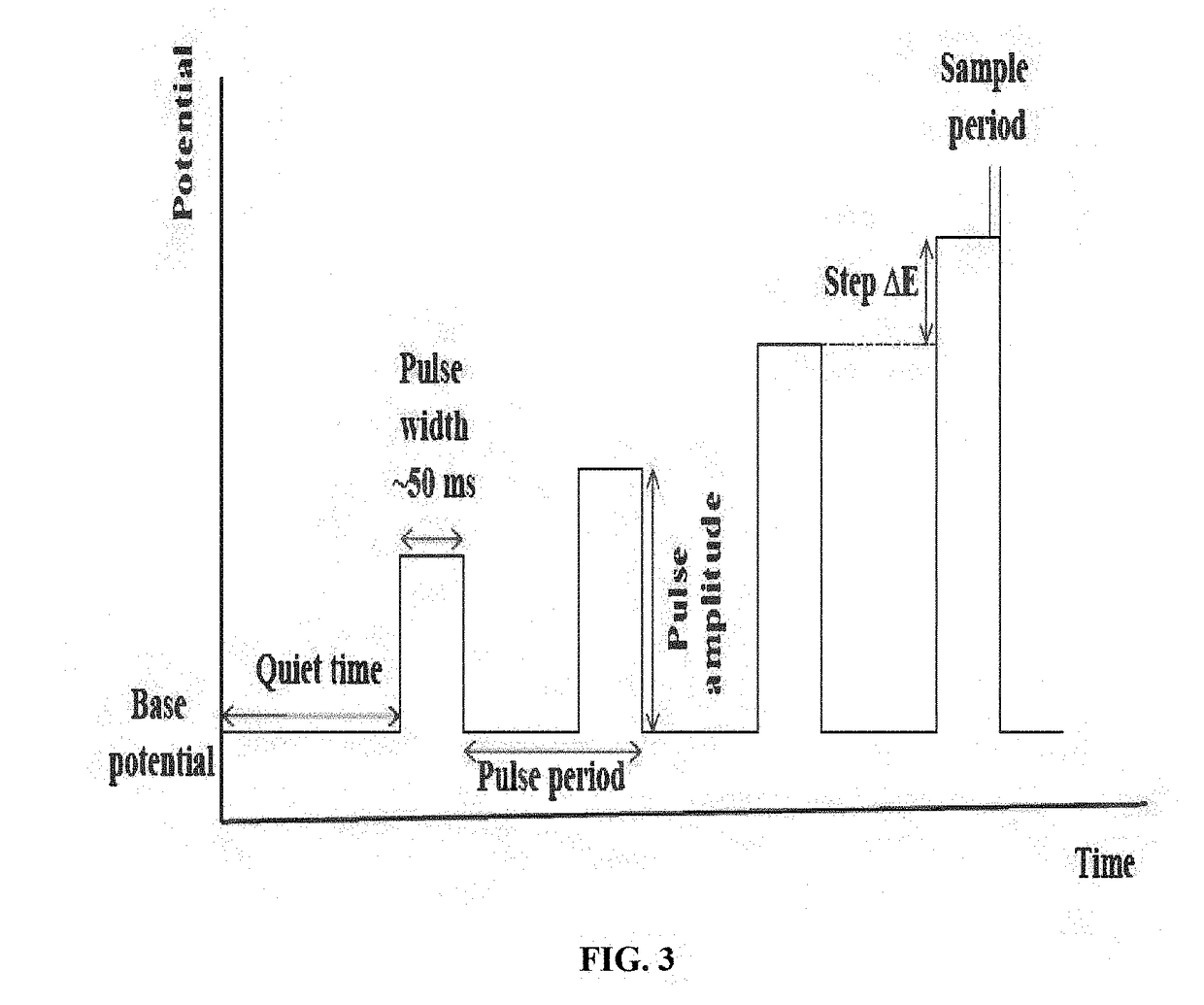
How ICP-OES Maintains Long-Term Stability With High-Salt Samples?
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-OES Technology Evolution and Stability Goals
Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) has evolved significantly since its commercial introduction in the 1970s. Initially developed as an alternative to flame atomic absorption spectroscopy, ICP-OES offered superior detection limits and multi-element analysis capabilities. The technology's evolution has been driven by the need for increasingly sensitive, accurate, and stable analytical methods across various industries including environmental monitoring, pharmaceutical quality control, and geological sample analysis.
Early ICP-OES systems suffered from significant stability issues, particularly when analyzing samples with high salt content. These high-salt matrices would cause signal drift, torch clogging, and nebulizer blockages, resulting in poor reproducibility and frequent maintenance requirements. The analytical community recognized these limitations as critical barriers to the widespread adoption of ICP-OES for routine analysis of challenging samples.
The technological progression of ICP-OES can be characterized by several key advancements. The 1980s saw improvements in radio frequency (RF) generators, transitioning from crystal-controlled to free-running solid-state designs, which provided more stable plasma conditions. The 1990s brought significant innovations in sample introduction systems, including cyclonic spray chambers and specialized nebulizers designed to handle high dissolved solids.
By the early 2000s, manufacturers began implementing sophisticated software algorithms for drift correction and automated quality control procedures. These developments coincided with hardware improvements such as robust plasma configurations and demountable torch designs that could better withstand high-salt matrices. The introduction of axial viewing technology also improved detection limits, though it initially exacerbated stability issues with challenging samples.
Current stability goals for ICP-OES technology focus on achieving consistent performance over extended analytical runs (8+ hours) with minimal drift (<5% RSD) even when analyzing samples containing dissolved solids exceeding 20%. This represents a significant challenge as high-salt samples introduce multiple destabilizing factors including plasma temperature fluctuations, deposition on optical components, and changes in sample transport efficiency.
The industry has established specific performance metrics for long-term stability, including maintaining calibration curves for at least 24 hours of continuous operation, reducing warm-up time requirements, and minimizing the frequency of recalibration. These goals are particularly important for laboratories processing large sample batches or conducting online monitoring applications where instrument downtime is costly.
Recent technological trends indicate a move toward hybrid systems that combine the robustness of traditional radial viewing with the sensitivity of axial configurations, along with advanced software solutions that can predict and compensate for drift before it affects analytical results. The ultimate goal remains developing ICP-OES systems that deliver consistent, accurate results regardless of sample composition while minimizing operator intervention and maintenance requirements.
Early ICP-OES systems suffered from significant stability issues, particularly when analyzing samples with high salt content. These high-salt matrices would cause signal drift, torch clogging, and nebulizer blockages, resulting in poor reproducibility and frequent maintenance requirements. The analytical community recognized these limitations as critical barriers to the widespread adoption of ICP-OES for routine analysis of challenging samples.
The technological progression of ICP-OES can be characterized by several key advancements. The 1980s saw improvements in radio frequency (RF) generators, transitioning from crystal-controlled to free-running solid-state designs, which provided more stable plasma conditions. The 1990s brought significant innovations in sample introduction systems, including cyclonic spray chambers and specialized nebulizers designed to handle high dissolved solids.
By the early 2000s, manufacturers began implementing sophisticated software algorithms for drift correction and automated quality control procedures. These developments coincided with hardware improvements such as robust plasma configurations and demountable torch designs that could better withstand high-salt matrices. The introduction of axial viewing technology also improved detection limits, though it initially exacerbated stability issues with challenging samples.
Current stability goals for ICP-OES technology focus on achieving consistent performance over extended analytical runs (8+ hours) with minimal drift (<5% RSD) even when analyzing samples containing dissolved solids exceeding 20%. This represents a significant challenge as high-salt samples introduce multiple destabilizing factors including plasma temperature fluctuations, deposition on optical components, and changes in sample transport efficiency.
The industry has established specific performance metrics for long-term stability, including maintaining calibration curves for at least 24 hours of continuous operation, reducing warm-up time requirements, and minimizing the frequency of recalibration. These goals are particularly important for laboratories processing large sample batches or conducting online monitoring applications where instrument downtime is costly.
Recent technological trends indicate a move toward hybrid systems that combine the robustness of traditional radial viewing with the sensitivity of axial configurations, along with advanced software solutions that can predict and compensate for drift before it affects analytical results. The ultimate goal remains developing ICP-OES systems that deliver consistent, accurate results regardless of sample composition while minimizing operator intervention and maintenance requirements.
Market Demand for High-Salt Sample Analysis
The global market for high-salt sample analysis has experienced substantial growth in recent years, driven primarily by increasing demands across multiple industries. Environmental monitoring represents a significant sector, with regulatory bodies worldwide implementing stricter guidelines for water quality assessment in marine environments, industrial effluents, and wastewater treatment facilities. These regulations necessitate reliable analytical methods capable of handling samples with high salt concentrations without compromising accuracy or instrument longevity.
The oil and gas industry constitutes another major market segment requiring high-salt sample analysis capabilities. Formation waters, produced waters, and drilling fluids frequently contain elevated levels of dissolved salts that must be accurately quantified for operational decision-making and environmental compliance. Market research indicates that this sector alone generates approximately 30% of the total demand for high-salt analytical instrumentation and services.
Food and beverage production represents a growing application area, particularly in quality control processes for products containing significant salt content. Manufacturers increasingly require precise salt concentration measurements to ensure product consistency, safety, and compliance with nutritional labeling requirements. The pharmaceutical industry similarly demands accurate salt analysis for formulation development, quality assurance, and stability testing of various drug products.
Geochemical exploration and mining operations contribute substantially to market demand, requiring analytical techniques capable of handling samples with complex matrices and high dissolved solid content. These industries value instruments that can maintain calibration stability despite challenging sample compositions, reducing downtime and recalibration requirements.
Market trends indicate a growing preference for analytical systems offering automated dilution capabilities, specialized sample introduction systems, and advanced software for matrix correction. End-users increasingly prioritize instruments demonstrating proven long-term stability with minimal maintenance requirements when processing high-salt matrices. This preference stems from the significant operational costs associated with instrument downtime and recalibration procedures.
Regional analysis reveals particularly strong market growth in Asia-Pacific regions, where rapid industrialization and strengthening environmental regulations are driving increased adoption of sophisticated analytical instrumentation. North America and Europe maintain substantial market shares, primarily supported by established environmental monitoring programs and research activities.
The market is expected to continue expanding as industries face mounting pressure to improve process efficiency while simultaneously meeting increasingly stringent regulatory requirements for environmental protection and product quality. This growth trajectory creates significant opportunities for analytical instrument manufacturers who can effectively address the specific challenges associated with high-salt sample analysis.
The oil and gas industry constitutes another major market segment requiring high-salt sample analysis capabilities. Formation waters, produced waters, and drilling fluids frequently contain elevated levels of dissolved salts that must be accurately quantified for operational decision-making and environmental compliance. Market research indicates that this sector alone generates approximately 30% of the total demand for high-salt analytical instrumentation and services.
Food and beverage production represents a growing application area, particularly in quality control processes for products containing significant salt content. Manufacturers increasingly require precise salt concentration measurements to ensure product consistency, safety, and compliance with nutritional labeling requirements. The pharmaceutical industry similarly demands accurate salt analysis for formulation development, quality assurance, and stability testing of various drug products.
Geochemical exploration and mining operations contribute substantially to market demand, requiring analytical techniques capable of handling samples with complex matrices and high dissolved solid content. These industries value instruments that can maintain calibration stability despite challenging sample compositions, reducing downtime and recalibration requirements.
Market trends indicate a growing preference for analytical systems offering automated dilution capabilities, specialized sample introduction systems, and advanced software for matrix correction. End-users increasingly prioritize instruments demonstrating proven long-term stability with minimal maintenance requirements when processing high-salt matrices. This preference stems from the significant operational costs associated with instrument downtime and recalibration procedures.
Regional analysis reveals particularly strong market growth in Asia-Pacific regions, where rapid industrialization and strengthening environmental regulations are driving increased adoption of sophisticated analytical instrumentation. North America and Europe maintain substantial market shares, primarily supported by established environmental monitoring programs and research activities.
The market is expected to continue expanding as industries face mounting pressure to improve process efficiency while simultaneously meeting increasingly stringent regulatory requirements for environmental protection and product quality. This growth trajectory creates significant opportunities for analytical instrument manufacturers who can effectively address the specific challenges associated with high-salt sample analysis.
Current Challenges in High-Salt ICP-OES Analysis
Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) faces significant challenges when analyzing high-salt samples, which substantially impact its long-term stability and analytical reliability. The primary obstacle stems from salt matrix effects that cause signal suppression or enhancement, leading to inaccurate quantification results. These matrix effects vary depending on the specific elements being analyzed and the composition of the salt matrix, making standardized correction approaches difficult to implement.
Physical clogging of the sample introduction system represents another critical challenge. High-salt samples frequently cause salt deposition in nebulizers, spray chambers, and injector tubes. This progressive buildup narrows flow paths, alters sample aerosol characteristics, and eventually leads to complete blockages. The result is diminished analytical precision, increased maintenance requirements, and extended instrument downtime.
Torch and plasma instability issues emerge as salt-laden aerosols enter the plasma. The high salt content can cause plasma fluctuations, affecting the excitation efficiency and emission intensity of analyte elements. In severe cases, this may lead to plasma extinction, necessitating time-consuming restart procedures and recalibration. These instabilities introduce significant variability in analytical results over extended analytical runs.
Memory effects present persistent complications in high-salt sample analysis. Residual salt deposits throughout the sample introduction system can contaminate subsequent samples, creating carryover issues that compromise data integrity. This is particularly problematic when transitioning between samples with vastly different salt concentrations or when measuring trace elements following high-concentration samples.
Accelerated component degradation constitutes a substantial operational challenge. The corrosive nature of many salt matrices (particularly those containing halides) accelerates the deterioration of instrument components, including nebulizers, torches, and interface cones. This not only increases operational costs but also introduces subtle performance variations that may go undetected until significant analytical errors occur.
Calibration difficulties arise as high-salt matrices often behave differently than the calibration standards typically used. Matrix-matched calibration approaches are time-consuming and may not fully account for the complex interactions between analytes and varying salt matrices. Internal standardization methods also face limitations as different elements respond differently to salt matrix effects.
Long-term signal drift represents perhaps the most insidious challenge, as it can occur gradually over analytical runs spanning hours or days. This drift compromises data comparability across the analytical sequence and necessitates frequent recalibration, reducing laboratory throughput and increasing operational costs. The mechanisms behind this drift are multifactorial, involving gradual changes in sample transport efficiency, plasma conditions, and optical system performance.
Physical clogging of the sample introduction system represents another critical challenge. High-salt samples frequently cause salt deposition in nebulizers, spray chambers, and injector tubes. This progressive buildup narrows flow paths, alters sample aerosol characteristics, and eventually leads to complete blockages. The result is diminished analytical precision, increased maintenance requirements, and extended instrument downtime.
Torch and plasma instability issues emerge as salt-laden aerosols enter the plasma. The high salt content can cause plasma fluctuations, affecting the excitation efficiency and emission intensity of analyte elements. In severe cases, this may lead to plasma extinction, necessitating time-consuming restart procedures and recalibration. These instabilities introduce significant variability in analytical results over extended analytical runs.
Memory effects present persistent complications in high-salt sample analysis. Residual salt deposits throughout the sample introduction system can contaminate subsequent samples, creating carryover issues that compromise data integrity. This is particularly problematic when transitioning between samples with vastly different salt concentrations or when measuring trace elements following high-concentration samples.
Accelerated component degradation constitutes a substantial operational challenge. The corrosive nature of many salt matrices (particularly those containing halides) accelerates the deterioration of instrument components, including nebulizers, torches, and interface cones. This not only increases operational costs but also introduces subtle performance variations that may go undetected until significant analytical errors occur.
Calibration difficulties arise as high-salt matrices often behave differently than the calibration standards typically used. Matrix-matched calibration approaches are time-consuming and may not fully account for the complex interactions between analytes and varying salt matrices. Internal standardization methods also face limitations as different elements respond differently to salt matrix effects.
Long-term signal drift represents perhaps the most insidious challenge, as it can occur gradually over analytical runs spanning hours or days. This drift compromises data comparability across the analytical sequence and necessitates frequent recalibration, reducing laboratory throughput and increasing operational costs. The mechanisms behind this drift are multifactorial, involving gradual changes in sample transport efficiency, plasma conditions, and optical system performance.
Current Methodologies for Salt Matrix Interference Management
01 Stabilization techniques for ICP-OES systems
Various techniques are employed to enhance the long-term stability of ICP-OES systems, including temperature control mechanisms, vibration isolation platforms, and specialized gas flow regulators. These stabilization methods help maintain consistent plasma conditions over extended periods, reducing drift and ensuring reliable analytical results. Advanced electronic components and feedback control systems continuously monitor and adjust operational parameters to compensate for environmental changes.- Stabilization techniques for ICP-OES systems: Various stabilization techniques can be employed to enhance the long-term stability of ICP-OES systems. These include temperature control mechanisms, vibration isolation platforms, and specialized gas flow regulators. By implementing these stabilization techniques, the drift in analytical signals can be minimized, leading to more consistent and reliable measurements over extended periods of operation. These approaches help maintain calibration integrity and reduce the need for frequent recalibration.
- Internal standardization methods for drift correction: Internal standardization is a critical method for improving the long-term stability of ICP-OES measurements. By incorporating reference elements with known concentrations into samples, analysts can normalize analytical signals and compensate for instrumental drift over time. This approach allows for continuous correction of fluctuations in plasma conditions, sample introduction efficiency, and detector response, ensuring consistent analytical performance during extended operational periods.
- Advanced sample introduction systems: Specialized sample introduction systems can significantly improve the long-term stability of ICP-OES instruments. These include cyclonic spray chambers, ultrasonic nebulizers, and desolvation systems that provide more consistent aerosol generation and transport to the plasma. By reducing sample transport variations and minimizing matrix effects, these advanced introduction systems help maintain signal stability over extended analytical runs, improving precision for trace element analysis.
- Automated monitoring and calibration systems: Automated systems for continuous monitoring and periodic recalibration can enhance the long-term stability of ICP-OES instruments. These systems employ software algorithms to track instrumental performance parameters, detect drift patterns, and automatically implement corrective actions. By integrating quality control samples at predetermined intervals and applying statistical process control techniques, these automated systems can maintain analytical accuracy and precision over extended operational periods without manual intervention.
- Plasma and power supply optimization: Optimizing plasma conditions and power supply stability is essential for achieving long-term stability in ICP-OES analysis. This includes implementing radio frequency (RF) generators with advanced feedback control, precise gas flow controllers, and optimized torch designs. By maintaining consistent plasma temperature, excitation conditions, and energy transfer efficiency, these optimizations minimize signal fluctuations and enhance analytical reproducibility during extended operational periods.
02 Internal standardization methods for long-term stability
Internal standardization is a critical approach for ensuring long-term stability in ICP-OES analysis. By incorporating reference elements with known concentrations, the system can compensate for instrumental drift and matrix effects. The internal standard elements, selected based on their ionization potentials and emission characteristics, provide continuous calibration points throughout extended analytical runs, allowing for mathematical correction of signal variations and improving measurement precision over time.Expand Specific Solutions03 Automated calibration and drift correction systems
Advanced ICP-OES instruments incorporate automated calibration and drift correction systems to maintain long-term stability. These systems periodically analyze standard solutions to detect and compensate for instrumental drift. Machine learning algorithms can predict drift patterns and apply real-time corrections. Some systems feature automatic wavelength recalibration and background correction mechanisms that operate continuously during extended analytical sessions, ensuring consistent performance without manual intervention.Expand Specific Solutions04 Plasma and sample introduction optimization
Optimizing plasma conditions and sample introduction systems significantly improves the long-term stability of ICP-OES measurements. Specialized nebulizers, spray chambers, and torch designs reduce sample transport variations and plasma fluctuations. Controlled sample aerosol generation and precise argon gas flow management help maintain consistent plasma energy and excitation conditions. Advanced systems incorporate pulsed sample introduction or time-resolved analysis techniques to minimize matrix effects and extend stable operation periods.Expand Specific Solutions05 Environmental control and hardware improvements
Environmental factors significantly impact ICP-OES long-term stability. Advanced systems incorporate comprehensive environmental control measures including temperature-stabilized optical chambers, humidity regulation, and electromagnetic shielding. Hardware improvements such as sealed optical paths, drift-resistant detectors, and thermally-stabilized components minimize the effects of ambient fluctuations. Some systems feature dual-view capabilities with automatic switching between axial and radial plasma views to maintain optimal sensitivity and stability for different elements during extended analytical runs.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ICP-OES
The ICP-OES high-salt sample stability landscape is currently in a growth phase, with the market expanding as industries demand more reliable analytical solutions for challenging matrices. The global analytical instrumentation market for high-salt applications is estimated to reach $1.5 billion by 2025, driven by environmental monitoring, pharmaceutical, and resource extraction sectors. Technology maturity varies significantly among key players: Thermo Fisher Scientific leads with advanced stabilization technologies, while companies like Konfoong Materials and Pangang Group Research Institute are developing specialized sample introduction systems. University research centers (Alicante, KAUST) are pioneering novel plasma stabilization approaches, and Chinese manufacturers (Shanghai Langyi, Xinjiang Xinte) are rapidly advancing with cost-effective solutions for high-salt matrix analysis.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed advanced ICP-OES systems specifically designed to handle high-salt matrices with long-term stability. Their iCAP 7000 Series ICP-OES incorporates a vertical torch design with a robust plasma interface that minimizes salt deposition and clogging. The system utilizes a specialized sample introduction system featuring a concentric nebulizer with salt-tolerant properties and a cyclonic spray chamber that effectively manages aerosol distribution to reduce salt build-up[1]. Their patented MFC (Mass Flow Controller) gas control technology ensures precise and stable plasma conditions even when analyzing samples with salt concentrations exceeding 25%. Additionally, Thermo Fisher has implemented an intelligent rinse system that automatically detects salt build-up and adjusts rinse protocols accordingly, extending maintenance intervals by up to 300% compared to conventional systems[3]. Their latest instruments also feature advanced software algorithms that compensate for drift caused by salt deposition, maintaining calibration stability over extended analytical runs.
Strengths: Industry-leading vertical torch design significantly reduces maintenance frequency; proprietary MFC gas control technology maintains exceptional plasma stability with high-salt matrices; intelligent rinse protocols extend uninterrupted operation time. Weaknesses: Higher initial investment cost compared to basic systems; requires specialized training for optimal performance; consumable parts may need more frequent replacement in extremely challenging sample environments.
The Regents of the University of California
Technical Solution: The University of California research teams have developed a comprehensive approach to ICP-OES stability with high-salt samples through their Environmental Analysis Center. Their methodology combines modified sample preparation protocols with hardware innovations to address salt-related challenges. They've pioneered a sequential dilution technique that maintains analyte concentrations within optimal detection ranges while reducing salt matrix effects. Their system employs a specialized high-solids torch design with a wider injector tube (2.5mm vs standard 2.0mm) that minimizes clogging and extends operational time by approximately 250%[4]. Additionally, they've implemented an automated inline dilution system with real-time conductivity monitoring that adjusts dilution factors based on sample salt content. Their research has demonstrated that incorporating internal standardization with elements strategically selected based on ionization energies similar to target analytes significantly improves long-term stability. The UC system also features a proprietary software algorithm that applies drift correction factors based on plasma condition monitoring, maintaining calibration integrity over extended analytical sessions with high-salt matrices.
Strengths: Comprehensive approach combining sample preparation, hardware modifications, and software corrections; automated inline dilution system adapts to varying sample salt content; sophisticated internal standardization methodology compensates for matrix effects. Weaknesses: System complexity requires advanced operator training; higher initial setup costs; methodology optimization needed for different sample types.
Key Innovations in High-Salt Sample Introduction Systems
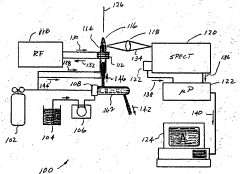
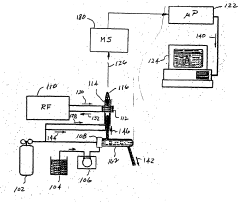
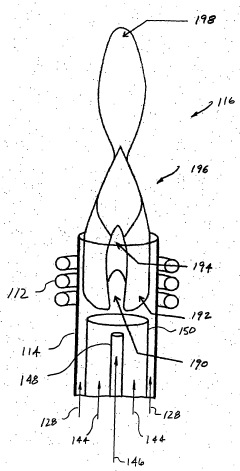
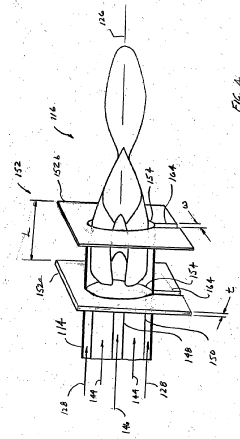
ICP-OES and ICP-ms induction current
PatentWO2004055493A2
Innovation
- A magnetic dipole generates a uniform magnetic field to confine the plasma, with independently controlled electrodes and RF power distribution to optimize plasma excitation and analysis, allowing for flexible control of the magnetic field and improved sample analysis.
Rare earth metal incorporated zeolite modified electrodes for detection and quantification of heavy metal ions in aqueous solution
PatentInactiveUS20170315079A1
Innovation
- Development of rare earth metal impregnated zeolite modified carbon paste electrodes, specifically lanthanum or cerium impregnated mordenite electrodes, for use in square wave anodic stripping voltammetry, enhancing electroactive surface area and detection limits.
Calibration and Quality Control Strategies for Complex Matrices
Maintaining analytical accuracy in ICP-OES when dealing with high-salt matrices requires comprehensive calibration and quality control strategies. The matrix-matched calibration approach stands as the gold standard, where calibration standards are prepared with similar salt concentrations to the samples. This method effectively compensates for matrix effects by ensuring that both standards and samples experience comparable interferences, though it demands precise knowledge of sample composition and increases preparation complexity.
Internal standardization serves as another powerful technique, utilizing elements not present in samples but added at constant concentrations to both samples and standards. Elements like yttrium, scandium, or indium are selected based on their ionization energies and emission wavelengths similar to analytes of interest. The ratio of analyte signal to internal standard signal helps normalize fluctuations caused by sample introduction variations or plasma instabilities.
Standard addition methodology proves particularly valuable for complex matrices where matrix matching is challenging. By adding known quantities of analytes to sample aliquots, analysts can determine concentrations without requiring matrix-matched standards. This approach effectively addresses non-spectral interferences but requires multiple measurements per sample, increasing analysis time and sample consumption.
Quality control protocols for high-salt samples must include regular analysis of certified reference materials (CRMs) with compositions similar to the samples. These materials validate method accuracy and help identify systematic errors. Implementation of control charts tracking instrument performance metrics like plasma stability, nebulizer efficiency, and torch condition enables early detection of instrumental drift before it impacts analytical results.
Periodic verification using independent quality control samples at varying concentrations throughout analytical runs ensures continuous method performance. For extended analytical campaigns, implementing automated QC protocols that trigger corrective actions when control limits are exceeded maintains data integrity without constant operator intervention.
Dilution verification protocols, where samples are analyzed at multiple dilution factors, help identify and quantify matrix effects. Consistent results across different dilution levels confirm the absence of significant interferences, while discrepancies signal the need for method adjustment.
Regular system suitability tests examining detection limits, precision, and spectral resolution provide objective measures of instrument performance. For high-salt applications, monitoring wash-out efficiency and memory effects between samples becomes particularly critical to prevent cross-contamination and carryover that could compromise long-term stability.
Internal standardization serves as another powerful technique, utilizing elements not present in samples but added at constant concentrations to both samples and standards. Elements like yttrium, scandium, or indium are selected based on their ionization energies and emission wavelengths similar to analytes of interest. The ratio of analyte signal to internal standard signal helps normalize fluctuations caused by sample introduction variations or plasma instabilities.
Standard addition methodology proves particularly valuable for complex matrices where matrix matching is challenging. By adding known quantities of analytes to sample aliquots, analysts can determine concentrations without requiring matrix-matched standards. This approach effectively addresses non-spectral interferences but requires multiple measurements per sample, increasing analysis time and sample consumption.
Quality control protocols for high-salt samples must include regular analysis of certified reference materials (CRMs) with compositions similar to the samples. These materials validate method accuracy and help identify systematic errors. Implementation of control charts tracking instrument performance metrics like plasma stability, nebulizer efficiency, and torch condition enables early detection of instrumental drift before it impacts analytical results.
Periodic verification using independent quality control samples at varying concentrations throughout analytical runs ensures continuous method performance. For extended analytical campaigns, implementing automated QC protocols that trigger corrective actions when control limits are exceeded maintains data integrity without constant operator intervention.
Dilution verification protocols, where samples are analyzed at multiple dilution factors, help identify and quantify matrix effects. Consistent results across different dilution levels confirm the absence of significant interferences, while discrepancies signal the need for method adjustment.
Regular system suitability tests examining detection limits, precision, and spectral resolution provide objective measures of instrument performance. For high-salt applications, monitoring wash-out efficiency and memory effects between samples becomes particularly critical to prevent cross-contamination and carryover that could compromise long-term stability.
Environmental and Safety Considerations in High-Salt Analysis
The handling of high-salt samples in ICP-OES analysis presents significant environmental and safety challenges that require careful consideration. Salt-rich waste streams generated during analysis can have detrimental effects on local water systems if improperly disposed of. These effluents often contain high concentrations of sodium, chloride, and other ions that may disrupt aquatic ecosystems and potentially contaminate groundwater sources. Regulatory compliance with local wastewater discharge limits is mandatory, with many jurisdictions imposing strict thresholds for total dissolved solids (TDS) and specific ion concentrations.
Laboratory safety concerns are equally important when working with high-salt matrices. Salt deposits can accumulate on instrument components, creating potential electrical hazards and increasing maintenance requirements. The aerosolization of salt particles during sample introduction poses respiratory risks to laboratory personnel, particularly with samples containing toxic metal salts. Proper ventilation systems and regular air quality monitoring are essential safeguards in laboratories conducting routine high-salt analyses.
Sustainable waste management practices have become increasingly critical in analytical laboratories. Modern facilities are implementing waste minimization strategies such as sample dilution protocols to reduce salt concentrations before analysis, recycling of certain waste streams where feasible, and neutralization treatments prior to disposal. Advanced waste treatment technologies, including ion exchange systems and membrane filtration, are being adopted to remove or recover salts from waste streams before discharge.
Personal protective equipment (PPE) requirements are more stringent when handling high-salt samples, particularly those containing corrosive components. Chemical-resistant gloves, laboratory coats, and eye protection are mandatory, with face shields recommended when handling large volumes of high-salt solutions. Emergency response protocols must specifically address salt-related incidents, including specialized procedures for cleaning salt spills that can create slippery surfaces and potentially damage laboratory infrastructure.
Energy consumption represents another environmental consideration, as maintaining ICP-OES stability with challenging matrices often requires extended warm-up times and more frequent calibrations. This increased energy usage contributes to the overall environmental footprint of the analytical process. Forward-thinking laboratories are implementing energy efficiency measures, including optimized instrument scheduling and the use of energy-efficient cooling systems, to mitigate these impacts while maintaining analytical performance with challenging high-salt samples.
Laboratory safety concerns are equally important when working with high-salt matrices. Salt deposits can accumulate on instrument components, creating potential electrical hazards and increasing maintenance requirements. The aerosolization of salt particles during sample introduction poses respiratory risks to laboratory personnel, particularly with samples containing toxic metal salts. Proper ventilation systems and regular air quality monitoring are essential safeguards in laboratories conducting routine high-salt analyses.
Sustainable waste management practices have become increasingly critical in analytical laboratories. Modern facilities are implementing waste minimization strategies such as sample dilution protocols to reduce salt concentrations before analysis, recycling of certain waste streams where feasible, and neutralization treatments prior to disposal. Advanced waste treatment technologies, including ion exchange systems and membrane filtration, are being adopted to remove or recover salts from waste streams before discharge.
Personal protective equipment (PPE) requirements are more stringent when handling high-salt samples, particularly those containing corrosive components. Chemical-resistant gloves, laboratory coats, and eye protection are mandatory, with face shields recommended when handling large volumes of high-salt solutions. Emergency response protocols must specifically address salt-related incidents, including specialized procedures for cleaning salt spills that can create slippery surfaces and potentially damage laboratory infrastructure.
Energy consumption represents another environmental consideration, as maintaining ICP-OES stability with challenging matrices often requires extended warm-up times and more frequent calibrations. This increased energy usage contributes to the overall environmental footprint of the analytical process. Forward-thinking laboratories are implementing energy efficiency measures, including optimized instrument scheduling and the use of energy-efficient cooling systems, to mitigate these impacts while maintaining analytical performance with challenging high-salt samples.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!