ICP-OES QC Protocols: CRMs, Spikes And Control Charts
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-OES QC Evolution and Objectives
Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) has evolved significantly since its commercial introduction in the 1970s. Initially developed as an alternative to flame atomic absorption spectroscopy, ICP-OES offered superior detection limits and multi-element analysis capabilities. The evolution of quality control protocols for this technology has paralleled advancements in instrumentation, analytical methodology, and regulatory requirements across various industries.
Early ICP-OES systems relied on rudimentary quality control measures, often limited to basic calibration procedures and occasional reference material checks. By the 1980s, as the technology gained wider adoption in environmental, pharmaceutical, and industrial applications, more structured QC protocols emerged. The introduction of certified reference materials (CRMs) during this period marked a significant milestone in standardizing analytical quality assurance.
The 1990s witnessed the integration of statistical process control principles into ICP-OES quality management, with control charts becoming standard practice in analytical laboratories. This period also saw the formalization of spike recovery tests as a means to assess matrix effects and validate method performance. These developments coincided with increasingly stringent regulatory frameworks in environmental monitoring, food safety, and pharmaceutical manufacturing.
The digital revolution of the early 2000s transformed ICP-OES QC protocols through automated data processing, real-time monitoring capabilities, and sophisticated statistical analysis tools. Laboratory information management systems (LIMS) enabled more comprehensive documentation and tracking of quality control parameters, facilitating compliance with evolving regulatory standards such as ISO/IEC 17025.
Current objectives in ICP-OES quality control focus on achieving greater accuracy, precision, and reliability while optimizing operational efficiency. Key goals include minimizing false positives/negatives, ensuring data comparability across different laboratories, and developing robust protocols that can withstand scrutiny in regulatory and legal contexts. The integration of artificial intelligence and machine learning algorithms represents the frontier in ICP-OES QC, with potential applications in automated anomaly detection and predictive maintenance.
Another critical objective is the harmonization of QC protocols across global regulatory frameworks, addressing challenges posed by differing regional standards. This includes establishing consensus on appropriate CRM selection criteria, spike recovery acceptance limits, and control chart interpretation guidelines. The development of matrix-matched reference materials for complex sample types remains a priority for improving method validation in challenging analytical scenarios.
The ultimate aim of modern ICP-OES QC protocols is to establish a comprehensive quality assurance framework that balances analytical rigor with practical laboratory constraints, ensuring defensible data generation while maintaining operational efficiency. This requires continuous refinement of existing protocols and adaptation to emerging analytical challenges and technological innovations.
Early ICP-OES systems relied on rudimentary quality control measures, often limited to basic calibration procedures and occasional reference material checks. By the 1980s, as the technology gained wider adoption in environmental, pharmaceutical, and industrial applications, more structured QC protocols emerged. The introduction of certified reference materials (CRMs) during this period marked a significant milestone in standardizing analytical quality assurance.
The 1990s witnessed the integration of statistical process control principles into ICP-OES quality management, with control charts becoming standard practice in analytical laboratories. This period also saw the formalization of spike recovery tests as a means to assess matrix effects and validate method performance. These developments coincided with increasingly stringent regulatory frameworks in environmental monitoring, food safety, and pharmaceutical manufacturing.
The digital revolution of the early 2000s transformed ICP-OES QC protocols through automated data processing, real-time monitoring capabilities, and sophisticated statistical analysis tools. Laboratory information management systems (LIMS) enabled more comprehensive documentation and tracking of quality control parameters, facilitating compliance with evolving regulatory standards such as ISO/IEC 17025.
Current objectives in ICP-OES quality control focus on achieving greater accuracy, precision, and reliability while optimizing operational efficiency. Key goals include minimizing false positives/negatives, ensuring data comparability across different laboratories, and developing robust protocols that can withstand scrutiny in regulatory and legal contexts. The integration of artificial intelligence and machine learning algorithms represents the frontier in ICP-OES QC, with potential applications in automated anomaly detection and predictive maintenance.
Another critical objective is the harmonization of QC protocols across global regulatory frameworks, addressing challenges posed by differing regional standards. This includes establishing consensus on appropriate CRM selection criteria, spike recovery acceptance limits, and control chart interpretation guidelines. The development of matrix-matched reference materials for complex sample types remains a priority for improving method validation in challenging analytical scenarios.
The ultimate aim of modern ICP-OES QC protocols is to establish a comprehensive quality assurance framework that balances analytical rigor with practical laboratory constraints, ensuring defensible data generation while maintaining operational efficiency. This requires continuous refinement of existing protocols and adaptation to emerging analytical challenges and technological innovations.
Market Demand for Analytical Quality Control
The analytical quality control market has witnessed substantial growth in recent years, driven primarily by increasing regulatory requirements across industries such as pharmaceuticals, environmental monitoring, food safety, and materials testing. The global market for analytical quality control solutions was valued at approximately 5.2 billion USD in 2022, with projections indicating a compound annual growth rate of 7.3% through 2028.
ICP-OES (Inductively Coupled Plasma-Optical Emission Spectrometry) quality control protocols represent a significant segment within this market, as organizations increasingly recognize the critical importance of ensuring accurate and reliable elemental analysis results. The demand for comprehensive QC protocols incorporating Certified Reference Materials (CRMs), spike recoveries, and statistical control charts has grown substantially, particularly in regulated industries where analytical data quality directly impacts compliance status and operational decisions.
Market research indicates that over 78% of analytical laboratories consider robust quality control systems essential for maintaining competitive advantage, with 65% specifically highlighting the need for improved ICP-OES quality control methodologies. This demand stems from several key factors: increasingly stringent regulatory requirements, growing complexity of sample matrices, higher expectations for detection limits, and the need for defensible data in litigation and regulatory submissions.
The pharmaceutical sector represents the largest market segment for ICP-OES quality control protocols, accounting for approximately 32% of the total market share. This is closely followed by environmental testing laboratories at 28%, mining and metallurgy at 18%, and food safety testing at 14%. The remaining market share is distributed across various industries including petrochemicals, semiconductors, and academic research.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate at 9.1% annually, driven by expanding industrial bases in China and India, coupled with strengthening regulatory frameworks for environmental protection and product quality.
Customer surveys reveal that laboratories are increasingly seeking integrated quality control solutions that combine appropriate reference materials, automated spike recovery procedures, and sophisticated statistical tools for trend analysis and early detection of analytical system drift. There is particular demand for software solutions that can automate control charting and provide real-time alerts when analytical systems deviate from established performance parameters.
ICP-OES (Inductively Coupled Plasma-Optical Emission Spectrometry) quality control protocols represent a significant segment within this market, as organizations increasingly recognize the critical importance of ensuring accurate and reliable elemental analysis results. The demand for comprehensive QC protocols incorporating Certified Reference Materials (CRMs), spike recoveries, and statistical control charts has grown substantially, particularly in regulated industries where analytical data quality directly impacts compliance status and operational decisions.
Market research indicates that over 78% of analytical laboratories consider robust quality control systems essential for maintaining competitive advantage, with 65% specifically highlighting the need for improved ICP-OES quality control methodologies. This demand stems from several key factors: increasingly stringent regulatory requirements, growing complexity of sample matrices, higher expectations for detection limits, and the need for defensible data in litigation and regulatory submissions.
The pharmaceutical sector represents the largest market segment for ICP-OES quality control protocols, accounting for approximately 32% of the total market share. This is closely followed by environmental testing laboratories at 28%, mining and metallurgy at 18%, and food safety testing at 14%. The remaining market share is distributed across various industries including petrochemicals, semiconductors, and academic research.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate at 9.1% annually, driven by expanding industrial bases in China and India, coupled with strengthening regulatory frameworks for environmental protection and product quality.
Customer surveys reveal that laboratories are increasingly seeking integrated quality control solutions that combine appropriate reference materials, automated spike recovery procedures, and sophisticated statistical tools for trend analysis and early detection of analytical system drift. There is particular demand for software solutions that can automate control charting and provide real-time alerts when analytical systems deviate from established performance parameters.
Current Challenges in ICP-OES Quality Control
Despite significant advancements in ICP-OES technology, laboratories continue to face substantial challenges in implementing robust quality control protocols. One primary challenge is the selection and availability of appropriate Certified Reference Materials (CRMs) that match complex sample matrices. Many laboratories struggle to find CRMs that adequately represent their specific sample types, particularly for environmental samples with variable compositions or pharmaceutical products with proprietary formulations. This matrix mismatch can lead to systematic errors in analytical results that may go undetected.
The preparation and maintenance of spike recovery samples present another significant hurdle. Analysts often encounter difficulties in determining optimal spike concentrations that balance detection capabilities with realistic representation of sample conditions. Additionally, spike stability issues arise when working with volatile elements or compounds that may degrade over time, compromising the reliability of recovery measurements and introducing bias into analytical results.
Control chart implementation and interpretation remain problematic areas for many laboratories. The statistical complexity of establishing meaningful control limits often leads to either overly restrictive or excessively lenient quality control parameters. Technicians frequently lack adequate training in statistical process control principles, resulting in misinterpretation of control chart signals and delayed responses to analytical system failures.
Instrument drift compensation represents a persistent technical challenge that affects QC protocol effectiveness. Modern ICP-OES systems, despite internal standardization capabilities, still exhibit temporal drift patterns that can be difficult to distinguish from actual sample variations. This complicates the establishment of reliable control limits and may necessitate frequent recalibration, reducing laboratory throughput and efficiency.
Data management and integration issues further compound quality control challenges. Many laboratories operate with disconnected systems where QC data is managed separately from routine analytical results, hindering comprehensive performance monitoring. The lack of integrated software solutions that can automatically flag QC failures and trigger appropriate corrective actions leads to delayed identification of analytical problems.
Regulatory compliance adds another layer of complexity, as different industries and regions maintain varying QC requirements for ICP-OES analyses. Laboratories serving multiple sectors must navigate conflicting standards and documentation requirements, often implementing redundant QC measures to satisfy all applicable regulations. This regulatory fragmentation increases operational costs and complicates standardization efforts across the analytical community.
The preparation and maintenance of spike recovery samples present another significant hurdle. Analysts often encounter difficulties in determining optimal spike concentrations that balance detection capabilities with realistic representation of sample conditions. Additionally, spike stability issues arise when working with volatile elements or compounds that may degrade over time, compromising the reliability of recovery measurements and introducing bias into analytical results.
Control chart implementation and interpretation remain problematic areas for many laboratories. The statistical complexity of establishing meaningful control limits often leads to either overly restrictive or excessively lenient quality control parameters. Technicians frequently lack adequate training in statistical process control principles, resulting in misinterpretation of control chart signals and delayed responses to analytical system failures.
Instrument drift compensation represents a persistent technical challenge that affects QC protocol effectiveness. Modern ICP-OES systems, despite internal standardization capabilities, still exhibit temporal drift patterns that can be difficult to distinguish from actual sample variations. This complicates the establishment of reliable control limits and may necessitate frequent recalibration, reducing laboratory throughput and efficiency.
Data management and integration issues further compound quality control challenges. Many laboratories operate with disconnected systems where QC data is managed separately from routine analytical results, hindering comprehensive performance monitoring. The lack of integrated software solutions that can automatically flag QC failures and trigger appropriate corrective actions leads to delayed identification of analytical problems.
Regulatory compliance adds another layer of complexity, as different industries and regions maintain varying QC requirements for ICP-OES analyses. Laboratories serving multiple sectors must navigate conflicting standards and documentation requirements, often implementing redundant QC measures to satisfy all applicable regulations. This regulatory fragmentation increases operational costs and complicates standardization efforts across the analytical community.
Standard QC Methodologies: CRMs, Spikes, Control Charts
01 Calibration and standardization methods for ICP-OES
Various calibration and standardization methods are employed to ensure accurate and reliable results in ICP-OES analysis. These methods include the use of reference standards, multi-point calibration curves, and internal standardization techniques. Proper calibration helps to compensate for matrix effects, instrument drift, and other factors that can affect measurement accuracy. Regular calibration verification is essential for maintaining the quality control of ICP-OES analytical procedures.- Calibration and standardization methods for ICP-OES: Various calibration and standardization methods are employed to ensure accurate and reliable results in ICP-OES analysis. These methods include the use of reference standards, multi-point calibration curves, and internal standardization techniques. Proper calibration helps to compensate for matrix effects, instrument drift, and other factors that can affect measurement accuracy. Regular calibration verification is essential for maintaining the quality control of ICP-OES analytical procedures.
- Sample preparation protocols for ICP-OES analysis: Effective sample preparation is critical for accurate ICP-OES analysis. This includes methods for digestion, dissolution, dilution, and matrix modification to ensure that samples are in an appropriate form for analysis. Standardized protocols for sample preparation help to minimize contamination, reduce matrix interferences, and improve the precision and accuracy of analytical results. Quality control measures during sample preparation include the use of procedural blanks, certified reference materials, and duplicate samples.
- Automated quality control systems for ICP-OES: Automated systems for quality control in ICP-OES analysis enhance efficiency, reduce human error, and improve data reliability. These systems include automated sample handling, real-time monitoring of instrument performance, and integrated data management solutions. Automated quality control procedures can include scheduled calibration checks, drift correction, and statistical process control. These systems often incorporate software that can flag anomalous results and trigger appropriate corrective actions.
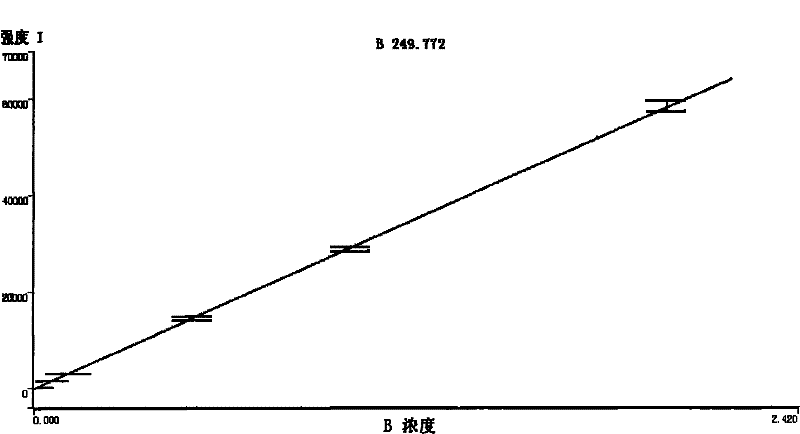
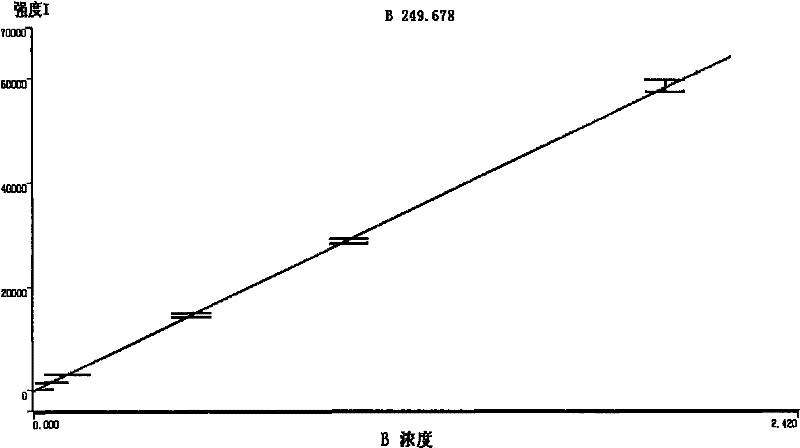
- Statistical methods for ICP-OES data validation: Statistical approaches are essential for validating ICP-OES analytical data and ensuring quality control. These methods include calculation of detection limits, quantification limits, precision estimates, and uncertainty measurements. Statistical tools are used to identify outliers, evaluate measurement bias, and assess the overall reliability of analytical results. Quality control charts, such as control charts for check standards and duplicate analyses, are commonly employed to monitor analytical performance over time and detect systematic errors.
- Interference management and correction techniques: Managing and correcting interferences is crucial for maintaining quality control in ICP-OES analysis. Spectral interferences, matrix effects, and physical interferences can significantly impact analytical results. Techniques for interference management include wavelength selection optimization, background correction methods, matrix matching, and the use of correction equations. Quality control protocols incorporate procedures for identifying potential interferences and validating correction methods to ensure accurate elemental determinations across diverse sample types.
02 Sample preparation protocols for ICP-OES analysis
Effective sample preparation is critical for accurate ICP-OES analysis. This includes methods for digestion, dissolution, dilution, and matrix modification to ensure that samples are in an appropriate form for analysis. Standardized protocols for sample preparation help to minimize contamination, reduce matrix interferences, and improve the precision and accuracy of analytical results. Quality control measures during sample preparation include the use of procedural blanks, certified reference materials, and duplicate samples.Expand Specific Solutions03 Automated quality control systems for ICP-OES
Automated systems for quality control in ICP-OES analysis enhance efficiency, reduce human error, and improve data reliability. These systems include automated sample handling, real-time monitoring of instrument performance, and integrated data management solutions. Automated QC protocols can include scheduled calibration checks, drift correction, and statistical process control. These systems often incorporate software that can flag anomalous results and trigger appropriate corrective actions, ensuring consistent analytical performance.Expand Specific Solutions04 Statistical methods for ICP-OES data validation
Statistical approaches are essential for validating ICP-OES analytical data and establishing quality control limits. These methods include calculation of detection limits, quantification limits, precision estimates, and uncertainty measurements. Statistical tools such as control charts, outlier tests, and trend analysis help monitor analytical performance over time. Proper statistical treatment of data ensures that analytical results meet required quality standards and that any deviations from expected performance are promptly identified and addressed.Expand Specific Solutions05 Interference management and correction techniques
Managing and correcting interferences is crucial for accurate ICP-OES analysis. Techniques include spectral interference correction algorithms, matrix matching, standard addition methods, and the use of collision/reaction cells. Effective interference management protocols involve systematic identification of potential interferences and implementation of appropriate correction strategies. Quality control procedures include regular verification of interference correction methods using synthetic mixtures and certified reference materials to ensure that analytical results remain accurate across different sample types and concentrations.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The ICP-OES QC protocols market is in a growth phase, driven by increasing demand for accurate analytical testing across industries. The market size is expanding due to stringent regulatory requirements in environmental monitoring, pharmaceuticals, and food safety. Technologically, the field is maturing with advanced automation and data management capabilities. Elemental Scientific leads with specialized sample introduction systems and automation solutions for ICP applications, while larger players like Hitachi, Panasonic, and Samsung incorporate these technologies into broader analytical portfolios. Academic institutions like Central South University contribute to protocol development. The competitive landscape features specialized instrumentation providers focusing on quality control innovations alongside established analytical equipment manufacturers who integrate ICP-OES capabilities within comprehensive laboratory solutions.
Elemental Scientific, Inc.
Technical Solution: Elemental Scientific has developed comprehensive ICP-OES QC protocols centered around their automated sample introduction systems. Their approach integrates precision-engineered nebulizers and spray chambers with automated quality control monitoring software. The company's SC-FAST system enables automated internal standard addition, calibration verification, and spike recoveries without manual intervention[1]. Their QC protocols emphasize the use of Certified Reference Materials (CRMs) at multiple concentration levels to establish traceability and validate method accuracy across the analytical range. For continuous monitoring, they've implemented automated control charts that track instrument drift, sensitivity changes, and background shifts in real-time, with customizable warning and action limits[2]. Their protocols also feature intelligent dilution capabilities that automatically address samples exceeding calibration ranges, ensuring data integrity without analyst intervention.
Strengths: Specialization in automated sample introduction systems provides superior precision and reduces human error in QC processes. Their integrated software solutions offer real-time monitoring capabilities that enhance data reliability. Weaknesses: Their systems typically require significant initial investment and specialized training, potentially limiting accessibility for smaller laboratories with budget constraints.
National Tobacco Quality Supervision and Inspection Center
Technical Solution: The National Tobacco Quality Supervision and Inspection Center has developed specialized ICP-OES QC protocols tailored for tobacco product analysis. Their approach centers on matrix-specific CRMs that accurately represent the complex organic composition of tobacco products. The Center's protocols implement a comprehensive spike recovery program at multiple stages of sample preparation to account for losses during ashing, digestion, and dilution processes[4]. Their control chart methodology incorporates both Shewhart and CUSUM (Cumulative Sum) techniques to detect both immediate shifts and gradual trends in analytical performance. For routine quality assurance, they've established a three-tier system of daily, weekly, and monthly verification procedures with increasingly stringent acceptance criteria. The Center has also pioneered specialized interference correction protocols to address the complex spectral interferences common in tobacco matrices, including automated correction factors derived from multi-element standard additions[5]. Their QC system integrates with a national database that allows comparison of performance metrics across multiple tobacco testing facilities.
Strengths: Highly specialized expertise in tobacco matrix analysis with protocols specifically optimized for challenging organic samples. Their multi-tier verification system provides exceptional long-term stability monitoring. Weaknesses: Protocols are narrowly focused on tobacco applications and may not transfer well to other sample types. Their specialized interference correction approaches require significant method development time.
Key Innovations in ICP-OES Quality Assurance
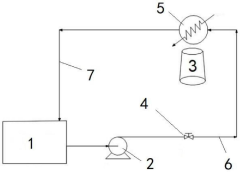
Volatile organic compound sampling system based on ICP-OES transformation
PatentActiveCN221124318U
Innovation
- Modify the ICP-OES instrument by adding a feed cooling unit, auxiliary oxygen system and atomizer cooling chamber support frame, increase the cooling unit and oxygen supply at the sample inlet, ensure sample stability and sufficient combustion, and flexibly adjust the coolant and oxygen amounts To adapt to different volatile organic compounds.
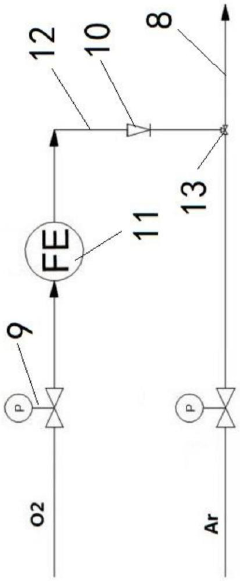
Method for measuring boric acid and borate in cosmetics by microwave digestion -ICP-OES
PatentInactiveCN101846629B
Innovation
- Integration of microwave digestion with ICP-OES for boric acid and borate detection in cosmetics, providing improved specificity and sensitivity compared to traditional methods.
- Use of yttrium as an internal standard element for response value correction, enhancing measurement accuracy by compensating for matrix effects and instrument drift.
- Optimized sample preparation using nitric acid and hydrogen peroxide for effective decomposition of organic substances in cosmetic samples.
Regulatory Compliance for Analytical Laboratories
Analytical laboratories face stringent regulatory requirements across various industries, particularly in pharmaceutical, environmental, and food safety sectors. ICP-OES (Inductively Coupled Plasma-Optical Emission Spectrometry) quality control protocols must align with these regulations to ensure compliance and data integrity. Major regulatory frameworks include FDA's 21 CFR Part 11, EPA Method 200.7, USP <233>, and ISO/IEC 17025, each imposing specific requirements for elemental analysis.
For pharmaceutical laboratories, compliance with ICH Q2(R1) guidelines is essential when implementing ICP-OES protocols. These guidelines mandate validation parameters including accuracy, precision, specificity, and robustness—all directly addressed through proper CRM (Certified Reference Material) selection and control charting procedures. The FDA's data integrity requirements further necessitate comprehensive audit trails for all QC activities.
Environmental testing laboratories must adhere to EPA methodologies which specify minimum QC requirements including initial demonstration of capability, method detection limit studies, and ongoing precision and recovery assessments. These regulations explicitly require the use of certified reference materials traceable to NIST standards and mandate specific spike recovery acceptance criteria.
Control charts represent a critical compliance tool across regulatory frameworks. Shewhart charts for instrument performance monitoring must be maintained with documented action and warning limits. Regulatory bodies increasingly expect statistical process control methodologies to be applied to analytical measurements, with control charts serving as primary evidence of continued method performance.
Documentation practices for CRMs and spike recoveries must follow strict protocols. Each reference material must have certificates of analysis maintained in laboratory records, with expiration dates tracked and documented. Spike recovery studies require formal documentation of preparation methodologies, acceptance criteria, and corrective actions when results fall outside established limits.
Regulatory inspections frequently focus on laboratory QC protocols, with particular emphasis on traceability of standards, appropriateness of control limits, and documentation of out-of-specification investigations. Recent FDA warning letters highlight deficiencies in elemental impurity testing, specifically citing inadequate system suitability testing and insufficient control charting practices for ICP-OES methods.
Compliance strategies must incorporate regular proficiency testing using blind samples, participation in interlaboratory comparison programs, and implementation of electronic data management systems with appropriate security features. These measures provide objective evidence of analytical competence while maintaining the data integrity requirements essential for regulatory compliance.
For pharmaceutical laboratories, compliance with ICH Q2(R1) guidelines is essential when implementing ICP-OES protocols. These guidelines mandate validation parameters including accuracy, precision, specificity, and robustness—all directly addressed through proper CRM (Certified Reference Material) selection and control charting procedures. The FDA's data integrity requirements further necessitate comprehensive audit trails for all QC activities.
Environmental testing laboratories must adhere to EPA methodologies which specify minimum QC requirements including initial demonstration of capability, method detection limit studies, and ongoing precision and recovery assessments. These regulations explicitly require the use of certified reference materials traceable to NIST standards and mandate specific spike recovery acceptance criteria.
Control charts represent a critical compliance tool across regulatory frameworks. Shewhart charts for instrument performance monitoring must be maintained with documented action and warning limits. Regulatory bodies increasingly expect statistical process control methodologies to be applied to analytical measurements, with control charts serving as primary evidence of continued method performance.
Documentation practices for CRMs and spike recoveries must follow strict protocols. Each reference material must have certificates of analysis maintained in laboratory records, with expiration dates tracked and documented. Spike recovery studies require formal documentation of preparation methodologies, acceptance criteria, and corrective actions when results fall outside established limits.
Regulatory inspections frequently focus on laboratory QC protocols, with particular emphasis on traceability of standards, appropriateness of control limits, and documentation of out-of-specification investigations. Recent FDA warning letters highlight deficiencies in elemental impurity testing, specifically citing inadequate system suitability testing and insufficient control charting practices for ICP-OES methods.
Compliance strategies must incorporate regular proficiency testing using blind samples, participation in interlaboratory comparison programs, and implementation of electronic data management systems with appropriate security features. These measures provide objective evidence of analytical competence while maintaining the data integrity requirements essential for regulatory compliance.
Data Management Systems for QC Implementation
Effective data management systems are crucial for implementing robust quality control protocols in ICP-OES analysis. Modern laboratories require integrated solutions that can handle the complex data generated from CRMs, spike recoveries, and control charts while ensuring regulatory compliance and operational efficiency.
Laboratory Information Management Systems (LIMS) specifically designed for analytical chemistry applications provide the backbone for QC data management. These systems offer automated data collection from ICP-OES instruments, reducing transcription errors and improving data integrity. Leading LIMS platforms include Thermo Scientific SampleManager, LabWare LIMS, and LabVantage, each offering specialized modules for spectroscopic data management.
Real-time monitoring capabilities represent a significant advancement in QC implementation. These systems continuously track instrument performance parameters and quality control metrics, allowing analysts to identify drift or calibration issues before they impact analytical results. Integration with control chart functionality enables automatic flagging when measurements exceed predetermined control limits, triggering appropriate corrective actions.
Statistical analysis packages embedded within data management systems provide sophisticated tools for evaluating QC data. These include trend analysis for long-term instrument performance, outlier detection algorithms, and measurement uncertainty calculations. Advanced systems incorporate machine learning algorithms that can predict potential instrument failures based on subtle changes in QC parameters, enabling proactive maintenance.
Cloud-based data management solutions offer additional advantages for multi-site operations. These platforms facilitate standardized QC protocols across different laboratory locations while providing centralized data storage and accessibility. This approach ensures consistent quality standards and enables organization-wide performance monitoring and benchmarking.
Data security and compliance features are essential components of modern QC data management systems. These include audit trails that document all changes to data, electronic signatures for result verification, and access controls that restrict data manipulation to authorized personnel. Such features ensure compliance with regulatory requirements such as ISO 17025, FDA 21 CFR Part 11, and various environmental monitoring standards.
Integration capabilities with enterprise resource planning (ERP) systems and electronic laboratory notebooks (ELN) create a seamless information flow throughout the organization. This integration allows QC data to inform broader operational decisions and facilitates knowledge sharing across departments, maximizing the value derived from analytical testing programs.
Laboratory Information Management Systems (LIMS) specifically designed for analytical chemistry applications provide the backbone for QC data management. These systems offer automated data collection from ICP-OES instruments, reducing transcription errors and improving data integrity. Leading LIMS platforms include Thermo Scientific SampleManager, LabWare LIMS, and LabVantage, each offering specialized modules for spectroscopic data management.
Real-time monitoring capabilities represent a significant advancement in QC implementation. These systems continuously track instrument performance parameters and quality control metrics, allowing analysts to identify drift or calibration issues before they impact analytical results. Integration with control chart functionality enables automatic flagging when measurements exceed predetermined control limits, triggering appropriate corrective actions.
Statistical analysis packages embedded within data management systems provide sophisticated tools for evaluating QC data. These include trend analysis for long-term instrument performance, outlier detection algorithms, and measurement uncertainty calculations. Advanced systems incorporate machine learning algorithms that can predict potential instrument failures based on subtle changes in QC parameters, enabling proactive maintenance.
Cloud-based data management solutions offer additional advantages for multi-site operations. These platforms facilitate standardized QC protocols across different laboratory locations while providing centralized data storage and accessibility. This approach ensures consistent quality standards and enables organization-wide performance monitoring and benchmarking.
Data security and compliance features are essential components of modern QC data management systems. These include audit trails that document all changes to data, electronic signatures for result verification, and access controls that restrict data manipulation to authorized personnel. Such features ensure compliance with regulatory requirements such as ISO 17025, FDA 21 CFR Part 11, and various environmental monitoring standards.
Integration capabilities with enterprise resource planning (ERP) systems and electronic laboratory notebooks (ELN) create a seamless information flow throughout the organization. This integration allows QC data to inform broader operational decisions and facilitates knowledge sharing across departments, maximizing the value derived from analytical testing programs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!